Molecular recognition of nucleosomes by binding partners
- PMID: 30991239
- PMCID: PMC6656623
- DOI: 10.1016/j.sbi.2019.03.010
Molecular recognition of nucleosomes by binding partners
Abstract
Nucleosomes represent the elementary units of chromatin packing and hubs in epigenetic signaling pathways. Across the chromatin and over the lifetime of the eukaryotic cell, nucleosomes experience a broad repertoire of alterations that affect their structure and binding with various chromatin factors. Dynamics of the histone core, nucleosomal and linker DNA, and intrinsic disorder of histone tails add further complexity to the nucleosome interaction landscape. In light of our understanding through the growing number of experimental and computational studies, we review the emerging patterns of molecular recognition of nucleosomes by their binding partners and assess the basic mechanisms of its regulation.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

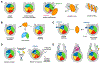

References
-
- Luger K, Mader AW, Richmond RK, Sargent DF, and Richmond TJ (1997). Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389, 251–260. - PubMed
-
- Liu X, Li M, Xia X, Li X, and Chen Z (2017). Mechanism of chromatin remodelling revealed by the Snf2-nucleosome structure. Nature 544, 440–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

