Cognizance of Molecular Methods for the Generation of Mutagenic Phage Display Antibody Libraries for Affinity Maturation
- PMID: 30991723
- PMCID: PMC6515083
- DOI: 10.3390/ijms20081861
Cognizance of Molecular Methods for the Generation of Mutagenic Phage Display Antibody Libraries for Affinity Maturation
Abstract
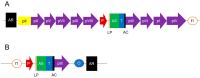
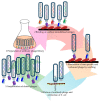
Antibodies leverage on their unique architecture to bind with an array of antigens. The strength of interaction has a direct relation to the affinity of the antibodies towards the antigen. In vivo affinity maturation is performed through multiple rounds of somatic hypermutation and selection in the germinal centre. This unique process involves intricate sequence rearrangements at the gene level via molecular mechanisms. The emergence of in vitro display technologies, mainly phage display and recombinant DNA technology, has helped revolutionize the way antibody improvements are being carried out in the laboratory. The adaptation of molecular approaches in vitro to replicate the in vivo processes has allowed for improvements in the way recombinant antibodies are designed and tuned. Combinatorial libraries, consisting of a myriad of possible antibodies, are capable of replicating the diversity of the natural human antibody repertoire. The isolation of target-specific antibodies with specific affinity characteristics can also be accomplished through modification of stringent protocols. Despite the ability to screen and select for high-affinity binders, some 'fine tuning' may be required to enhance antibody binding in terms of its affinity. This review will provide a brief account of phage display technology used for antibody generation followed by a summary of different combinatorial library characteristics. The review will focus on available strategies, which include molecular approaches, next generation sequencing, and in silico approaches used for antibody affinity maturation in both therapeutic and diagnostic applications.
Keywords: affinity maturation; combinatorial libraries; human monoclonal antibodies; phage display.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A review of in vitro stochastic and non-stochastic affinity maturation strategies for phage display derived monoclonal antibodies.Int J Biol Macromol. 2024 Oct;277(Pt 2):134217. doi: 10.1016/j.ijbiomac.2024.134217. Epub 2024 Jul 26. Int J Biol Macromol. 2024. PMID: 39069045 Review.
-
Facile Affinity Maturation of Single-Domain Antibodies Using Next-Generation DNA Sequencing.Methods Mol Biol. 2022;2446:245-268. doi: 10.1007/978-1-0716-2075-5_12. Methods Mol Biol. 2022. PMID: 35157277
-
Rapid Affinity Maturation of Novel Anti-PD-L1 Antibodies by a Fast Drop of the Antigen Concentration and FACS Selection of Yeast Libraries.Biomed Res Int. 2019 Dec 28;2019:6051870. doi: 10.1155/2019/6051870. eCollection 2019. Biomed Res Int. 2019. PMID: 31976323 Free PMC article.
-
Development, High-Throughput Profiling, and Biopanning of a Large Phage Display Single-Domain Antibody Library.Int J Mol Sci. 2024 Apr 27;25(9):4791. doi: 10.3390/ijms25094791. Int J Mol Sci. 2024. PMID: 38732011 Free PMC article.
-
Mammalian cell display and somatic hypermutation in vitro for human antibody discovery.Curr Drug Discov Technol. 2014 Mar;11(1):56-64. doi: 10.2174/15701638113109990037. Curr Drug Discov Technol. 2014. PMID: 23978037 Review.
Cited by
-
Structure and development of single domain antibodies as modules for therapeutics and diagnostics.Exp Biol Med (Maywood). 2019 Dec;244(17):1568-1576. doi: 10.1177/1535370219881129. Epub 2019 Oct 9. Exp Biol Med (Maywood). 2019. PMID: 31594404 Free PMC article. Review.
-
How repertoire data are changing antibody science.J Biol Chem. 2020 Jul 17;295(29):9823-9837. doi: 10.1074/jbc.REV120.010181. Epub 2020 May 14. J Biol Chem. 2020. PMID: 32409582 Free PMC article. Review.
-
The power of phages: revolutionizing cancer treatment.Front Oncol. 2023 Nov 15;13:1290296. doi: 10.3389/fonc.2023.1290296. eCollection 2023. Front Oncol. 2023. PMID: 38033486 Free PMC article. Review.
-
Development and experimental validation of computational methods for human antibody affinity enhancement.Brief Bioinform. 2024 Sep 23;25(6):bbae488. doi: 10.1093/bib/bbae488. Brief Bioinform. 2024. PMID: 39358035 Free PMC article.
-
Peptides, Antibodies, Peptide Antibodies and More.Int J Mol Sci. 2019 Dec 13;20(24):6289. doi: 10.3390/ijms20246289. Int J Mol Sci. 2019. PMID: 31847088 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

