Differential expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic sites in renal cell carcinoma
- PMID: 30992011
- PMCID: PMC6469103
- DOI: 10.1186/s12885-019-5578-4
Differential expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic sites in renal cell carcinoma
Abstract
Background: In clinical practice, the detection of biomarkers is mostly based on primary tumors for its convenience in acquisition. However, immune checkpoints may express differently between primary and metastatic tumor. Therefore, we aimed to compare the differential expressions of PD-1, PD-L1 and PD-L2 between the primary and metastatic sites of renal cell carcinoma (RCC).
Methods: Patients diagnosed with RCC by resection or fine needle aspiration of metastasis were included. Immunohistochemistry (IHC) was applied to detect PD-1, PD-L1 and PD-L2 expressions. SPSS 22.0 was applied to conduct Chi-square, consistency tests and Cox's proportional hazards regression models. GraphPad Prism 6 was used to plot survival curves and R software was used to calculate Predictive accuracy (PA).
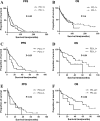
Results: In the whole cohort (N = 163), IHC results suggested a higher detection rate of PD-L1 in the metastasis than that of the primary site (χ2 = 4.66, p = 0.03), with a low consistent rate of 32.5%. Among different metastatic tumors, PD-1 was highly expressed in the lung/lymph node (65.3%) and poorly expressed in the brain (10.5%) and visceral metastases (12.5%). PD-L1 was highly expressed in lung/lymph node (37.5%) and the bone metastases (12.2%) on the contrary. In terms of survival analysis, patients with PD-1 expression either in the primary or metastasis had a shorter overall survival (OS) (HR: 1.59, 95% CI 1.08-2.36, p = 0.02). Also, PD-L1 expression in the primary was associated with a shorter OS (HR 2.55, 95% CI 1.06-6.15, p = 0.04). In the multivariate analysis, the predictive accuracy of the whole model for PFS was increased from 0.683 to 0.699 after adding PD-1.
Conclusion: PD-1, PD-L1 and PD-L2 were differentially expressed between primary and metastatic tumors. Histopathological examination of these immune check points in metastatic lesions of mRCC should be noticed, and its accurate diagnosis may be one of the effective ways to realize the individualized treatment.
Keywords: Differential expression; Immunological checkpoint; Metastases; Primary tumor; Renal cell carcinoma.
Conflict of interest statement
Ethics approval and consent to participate
The protocol of the present study, involving human clinical samples, was approved by the Ethics Committee of West China Hospital, Sichuan University. Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Differential expression of TIM-3 between primary and metastatic sites in renal cell carcinoma.BMC Cancer. 2019 Jan 10;19(1):49. doi: 10.1186/s12885-019-5273-5. BMC Cancer. 2019. PMID: 30630458 Free PMC article.
-
The Association Between PD-L1 Expression and the Clinical Outcomes to Vascular Endothelial Growth Factor-Targeted Therapy in Patients With Metastatic Clear Cell Renal Cell Carcinoma.Oncologist. 2015 Nov;20(11):1253-60. doi: 10.1634/theoncologist.2015-0151. Epub 2015 Sep 30. Oncologist. 2015. PMID: 26424759 Free PMC article.
-
Prognostic value of programmed death-1, programmed death-ligand 1, programmed death-ligand 2 expression, and CD8(+) T cell density in primary tumors and metastatic lymph nodes from patients with stage T1-4N+M0 gastric adenocarcinoma.Chin J Cancer. 2017 Jul 29;36(1):61. doi: 10.1186/s40880-017-0226-3. Chin J Cancer. 2017. PMID: 28754154 Free PMC article.
-
Factors Modifying the Associations of Single or Combination Programmed Cell Death 1 and Programmed Cell Death Ligand 1 Inhibitor Therapies With Survival Outcomes in Patients With Metastatic Clear Cell Renal Cell Carcinoma: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 Jan 4;4(1):e2034201. doi: 10.1001/jamanetworkopen.2020.34201. JAMA Netw Open. 2021. PMID: 33496794 Free PMC article.
-
PD-L1 Expression in Mastocytosis.Int J Mol Sci. 2019 May 13;20(9):2362. doi: 10.3390/ijms20092362. Int J Mol Sci. 2019. PMID: 31086024 Free PMC article. Review.
Cited by
-
Prognostic Impact of PD-1 and Tim-3 Expression in Tumor Tissue in Stage I-III Colorectal Cancer.Biomed Res Int. 2020 May 14;2020:5294043. doi: 10.1155/2020/5294043. eCollection 2020. Biomed Res Int. 2020. PMID: 32509862 Free PMC article.
-
Soluble PD-L1: a potential dynamic predictive biomarker for immunotherapy in patients with proficient mismatch repair colorectal cancer.J Transl Med. 2023 Jan 13;21(1):25. doi: 10.1186/s12967-023-03879-0. J Transl Med. 2023. PMID: 36639643 Free PMC article.
-
Programmed cell death-ligand 2: A neglected but important target in the immune response to cancer?Transl Oncol. 2020 Oct;13(10):100811. doi: 10.1016/j.tranon.2020.100811. Epub 2020 Jul 1. Transl Oncol. 2020. PMID: 32622310 Free PMC article. Review.
-
A model for predicting clinical prognosis based on brain metastasis-related genes in patients with breast cancer.Transl Cancer Res. 2023 Dec 31;12(12):3453-3470. doi: 10.21037/tcr-23-1123. Epub 2023 Dec 7. Transl Cancer Res. 2023. PMID: 38192988 Free PMC article.
-
Divulging a Pleiotropic Role of Succinate Receptor SUCNR1 in Renal Cell Carcinoma Microenvironment.Cancers (Basel). 2022 Dec 9;14(24):6064. doi: 10.3390/cancers14246064. Cancers (Basel). 2022. PMID: 36551549 Free PMC article.
References
-
- McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23(1):133–141. doi: 10.1200/JCO.2005.03.206. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

