Hepatitis C screening in hospitals: find the missing patients
- PMID: 30992019
- PMCID: PMC6469068
- DOI: 10.1186/s12985-019-1157-1
Hepatitis C screening in hospitals: find the missing patients
Abstract
Background: Hepatitis C virus (HCV) infection is one of the leading causes of liver cancer, creating enormous economic and social burdens. The Chinese government recommends routine screening of inpatients for HCV before invasive procedures to prevent iatric infections. However, the diagnosis and treatment rates for HCV remain low. The aim of this study was to use available routine screening data to understand the HCV screening of inpatients in different regions of China.
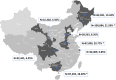
Methods: Inpatient information and HCV screening results were collected from January 2016 to December 2016 at eight tertiary hospitals in different regions of China to compare the HCV-positivity of hospitalized patients among different regions and age groups.
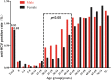
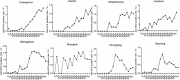
Results: The HCV screening rate of inpatients was more than 50%. A total of 467,008 inpatients were enrolled in the study (51.20% were male), and the HCV antibody (anti-HCV) -positive rate was 0.88% (95% confidence interval [CI], 0.85-0.91%) among the total population. This rate was significantly higher among all males compared with all females (0.91% vs 0.85%). Moreover, the HCV antibody-positive rate increased with age and was highest for the 60-64-year age group. Notably, 90.14% (3722/4129) of the anti-HCV seropositive patients were 40 years of age or older. HCV screening for people over 40 years old is recommended.
Conclusions: This study highlights the key role of routine examination for HCV infection in hospitalized patients. Full use of inpatient screening results to manage HCV antibody-positive patients and a screening strategy targeting inpatients 40 years and older were found to be low-cost and effective, which will help to find the missing millions of yet unaware patients and also accelerate the elimination of HCV in China.
Keywords: Hepatitis C virus; Hospital; Positive rate; Screening.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Frist Hospital of Jilin University (No.2017–379).
Consent for publication
All authors approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
HBsAg and anti-HCV screening in elderly hospitalized patients of a German tertiary referral centre.Z Gastroenterol. 2016 Mar;54(3):231-7. doi: 10.1055/s-0041-106656. Epub 2016 Apr 4. Z Gastroenterol. 2016. PMID: 27043886
-
A Seroprevalence Study of Hepatitis B and C Virus Infections in a Hospitalized Population in Romania, an Opportunity for a Better National Prevention and Control Strategy.J Gastrointestin Liver Dis. 2016 Mar;25(1):25-32. doi: 10.15403/jgld.2014.1121.251.hbc. J Gastrointestin Liver Dis. 2016. PMID: 27014751
-
Prevalence and characteristics of hepatitis C virus infection in Shenyang City, Northeast China, and prediction of HCV RNA positivity according to serum anti-HCV level: retrospective review of hospital data.Virol J. 2020 Mar 16;17(1):36. doi: 10.1186/s12985-020-01316-y. Virol J. 2020. PMID: 32178702 Free PMC article.
-
Evaluation of Hepatitis C Screening and Treatment Among Psychiatry Inpatients.J Clin Psychiatry. 2023 Jul 31;84(5):22m14623. doi: 10.4088/JCP.22m14623. J Clin Psychiatry. 2023. PMID: 37530609 Review.
-
Population-based screening of hepatitis C virus in the United States.Curr Opin Gastroenterol. 2019 May;35(3):177-182. doi: 10.1097/MOG.0000000000000520. Curr Opin Gastroenterol. 2019. PMID: 30844892 Free PMC article. Review.
Cited by
-
Hepatitis C virus micro-elimination: Where do we stand?World J Gastroenterol. 2021 Apr 28;27(16):1728-1737. doi: 10.3748/wjg.v27.i16.1728. World J Gastroenterol. 2021. PMID: 33967553 Free PMC article. Review.
-
Elimination of hepatitis C in a hospital characterized by infectious diseases.Front Public Health. 2023 Mar 16;11:1093578. doi: 10.3389/fpubh.2023.1093578. eCollection 2023. Front Public Health. 2023. PMID: 37006527 Free PMC article.
-
Chronic Hepatitis C Virus Infection: An Ongoing Challenge in Screening and Treatment.Life (Basel). 2023 Sep 26;13(10):1964. doi: 10.3390/life13101964. Life (Basel). 2023. PMID: 37895346 Free PMC article. Review.
-
Prevalence, diagnosis, and treatment of hepatitis C in Mainland China.Glob Health Med. 2021 Oct 31;3(5):270-275. doi: 10.35772/ghm.2021.01080. Glob Health Med. 2021. PMID: 34782868 Free PMC article. Review.
-
A scoping review on HCV screening strategies: population to screen and the test types.BMC Public Health. 2025 Jul 30;25(1):2589. doi: 10.1186/s12889-025-23809-7. BMC Public Health. 2025. PMID: 40739221 Free PMC article.
References
-
- Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. - DOI - PMC - PubMed
-
- Tanaka J, Yoshizawa H. A national project for the management of viral hepatitis toward prevention of hepatocellular carcinoma in Japan. Gan To Kagaku Ryoho. 2004;31:864–870. - PubMed
-
- Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

