Hepatitis C screening in hospitals: find the missing patients
- PMID: 30992019
- PMCID: PMC6469068
- DOI: 10.1186/s12985-019-1157-1
Hepatitis C screening in hospitals: find the missing patients
Abstract
Background: Hepatitis C virus (HCV) infection is one of the leading causes of liver cancer, creating enormous economic and social burdens. The Chinese government recommends routine screening of inpatients for HCV before invasive procedures to prevent iatric infections. However, the diagnosis and treatment rates for HCV remain low. The aim of this study was to use available routine screening data to understand the HCV screening of inpatients in different regions of China.
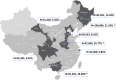
Methods: Inpatient information and HCV screening results were collected from January 2016 to December 2016 at eight tertiary hospitals in different regions of China to compare the HCV-positivity of hospitalized patients among different regions and age groups.
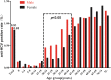
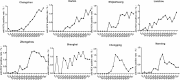
Results: The HCV screening rate of inpatients was more than 50%. A total of 467,008 inpatients were enrolled in the study (51.20% were male), and the HCV antibody (anti-HCV) -positive rate was 0.88% (95% confidence interval [CI], 0.85-0.91%) among the total population. This rate was significantly higher among all males compared with all females (0.91% vs 0.85%). Moreover, the HCV antibody-positive rate increased with age and was highest for the 60-64-year age group. Notably, 90.14% (3722/4129) of the anti-HCV seropositive patients were 40 years of age or older. HCV screening for people over 40 years old is recommended.
Conclusions: This study highlights the key role of routine examination for HCV infection in hospitalized patients. Full use of inpatient screening results to manage HCV antibody-positive patients and a screening strategy targeting inpatients 40 years and older were found to be low-cost and effective, which will help to find the missing millions of yet unaware patients and also accelerate the elimination of HCV in China.
Keywords: Hepatitis C virus; Hospital; Positive rate; Screening.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Frist Hospital of Jilin University (No.2017–379).
Consent for publication
All authors approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. - DOI - PMC - PubMed
-
- Tanaka J, Yoshizawa H. A national project for the management of viral hepatitis toward prevention of hepatocellular carcinoma in Japan. Gan To Kagaku Ryoho. 2004;31:864–870. - PubMed
-
- Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

