Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature
- PMID: 30992053
- PMCID: PMC6469201
- DOI: 10.1186/s40425-019-0585-1
Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature
Erratum in
-
Corrections to: Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature.J Immunother Cancer. 2019 Jun 24;7(1):158. doi: 10.1186/s40425-019-0639-4. J Immunother Cancer. 2019. PMID: 31234936 Free PMC article.
Abstract
Background: Checkpoint inhibitors (CPIs) have revolutionized the treatment of cancer, but their use remains limited by off-target inflammatory and immune-related adverse events. Solid organ transplantation (SOT) recipients have been excluded from clinical trials owing to concerns about alloimmunity, organ rejection, and immunosuppressive therapy. Thus, we conducted a retrospective study and literature review to evaluate the safety of CPIs in patients with cancer and prior SOT.
Methods: Data were collected from the medical records of patients with cancer and prior SOT who received CPIs at The University of Texas MD Anderson Cancer Center from January 1, 2004, through March 31, 2018. Additionally, we systematically reviewed five databases through April 2018 to identify studies reporting CPIs to treat cancer in SOT recipients. We evaluated the safety of CPIs in terms of alloimmunity, immune-related adverse events, and mortality. We also evaluated tumor response to CPIs.
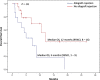
Results: Thirty-nine patients with allograft transplantation were identified. The median age was 63 years (range 14-79 years), 74% were male, 62% had metastatic melanoma, 77% received anti-PD-1 agents, and 59% had prior renal transplantation, 28% hepatic transplantation, and 13% cardiac transplantation. Median time to CPI initiation after SOT was 9 years (range 0.92-32 years). Allograft rejection occurred in 41% of patients (11/23 renal, 4/11 hepatic, and 1/5 cardiac transplantations), at similar rates for anti-CTLA-4 and anti-PD-1 therapy. The median time to rejection was 21 days (95% confidence interval 19.3-22.8 days). There were no associations between time since SOT and frequency, timing, or type of rejection. Overall, 31% of patients permanently discontinued CPIs because of allograft rejection. Graft loss occurred in 81%, and death was reported in 46%. Of the 12 patients with transplantation biopsies, nine (75%) had acute rejection, and five of these rejections were T cell-mediated. In melanoma patients, 36% responded to CPIs.
Conclusions: SOT recipients had a high allograft rejection rate that was observed shortly after CPI initiation, with high mortality rates. Further studies are needed to optimize the anticancer treatment approach in these patients.
Keywords: Alloimmunity; Cancer; Checkpoint inhibitors; Solid organ transplantation.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. IRB number: PA15–0071.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1. - DOI - PubMed
-
- Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. doi: 10.1016/S1470-2045(16)30364-3. - DOI - PMC - PubMed
-
- Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119–3125. doi: 10.1200/JCO.2016.67.9761. - DOI - PMC - PubMed
-
- Ribas Antoni, Hodi F. Stephen, Kefford Richard, Hamid Omid, Daud Adil, Wolchok Jedd D., Hwu Wen-Jen, Gangadhar Tara C., Patnaik Amita, Joshua Anthony M., Hersey Peter, Weber Jeffrey S., Dronca Roxana Stefania, Zarour Hassane M., Gergich Kevin, Li Xiaoyun (Nicole), Iannone Robert, Kang Soonmo Peter, Ebbinghaus Scot, Robert Caroline. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL) Journal of Clinical Oncology. 2014;32(18_suppl):LBA9000–LBA9000. doi: 10.1200/jco.2014.32.18_suppl.lba9000. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous