DNMT and HDAC inhibitors modulate MMP-9-dependent H3 N-terminal tail proteolysis and osteoclastogenesis
- PMID: 30992059
- PMCID: PMC6466663
- DOI: 10.1186/s13072-019-0270-0
DNMT and HDAC inhibitors modulate MMP-9-dependent H3 N-terminal tail proteolysis and osteoclastogenesis
Abstract
Background: MMP-9-dependent proteolysis of histone H3 N-terminal tail (H3NT) is an important mechanism for activation of gene expression during osteoclast differentiation. Like other enzymes targeting their substrates within chromatin structure, MMP-9 enzymatic activity toward H3NT is tightly controlled by histone modifications such as H3K18 acetylation (H3K18ac) and H3K27 monomethylation (H3K27me1). Growing evidence indicates that DNA methylation is another epigenetic mechanism controlling osteoclastogenesis, but whether DNA methylation is also critical for regulating MMP-9-dependent H3NT proteolysis and gene expression remains unknown.
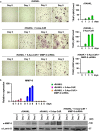
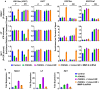
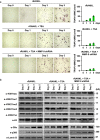
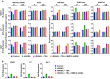
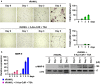
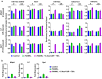
Results: We show here that treating RANKL-induced osteoclast progenitor (OCP) cells with the DNMT inhibitor 5-Aza-2'-deoxycytidine (5-Aza-CdR) induces CpG island hypomethylation and facilitates MMP-9 transcription. This increase in MMP-9 expression results in a significant enhancement of H3NT proteolysis and OCP cell differentiation. On the other hand, despite an increase in levels of H3K18ac, treatment with the HDAC inhibitor trichostatin A (TSA) leads to impairment of osteoclastogenic gene expression. Mechanistically, TSA treatment of OCP-induced cells stimulates H3K27ac with accompanying reduction in H3K27me1, which is a key modification to facilitate stable interaction of MMP-9 with nucleosomes for H3NT proteolysis. Moreover, hypomethylated osteoclastogenic genes in 5-Aza-CdR-treated cells remain transcriptionally inactive after TSA treatment, because H3K27 is highly acetylated and cannot be modified by G9a.
Conclusions: These findings clearly indicate that DNA methylation and histone modification are important mechanisms in regulating osteoclastogenic gene expression and that their inhibitors can be used as potential therapeutic tools for treating bone disorders.
Keywords: 5-Aza-dC; DNA methylation; H3 proteolysis; MMP-9; Osteoclast differentiation; TSA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
H3K27me1 is essential for MMP-9-dependent H3N-terminal tail proteolysis during osteoclastogenesis.Epigenetics Chromatin. 2018 May 28;11(1):23. doi: 10.1186/s13072-018-0193-1. Epigenetics Chromatin. 2018. PMID: 29807539 Free PMC article.
-
MMP-9 facilitates selective proteolysis of the histone H3 tail at genes necessary for proficient osteoclastogenesis.Genes Dev. 2016 Jan 15;30(2):208-19. doi: 10.1101/gad.268714.115. Epub 2016 Jan 7. Genes Dev. 2016. PMID: 26744418 Free PMC article.
-
MMP-9-dependent proteolysis of the histone H3 N-terminal tail: a critical epigenetic step in driving oncogenic transcription and colon tumorigenesis.Mol Oncol. 2024 Aug;18(8):2001-2019. doi: 10.1002/1878-0261.13652. Epub 2024 Apr 10. Mol Oncol. 2024. PMID: 38600695 Free PMC article.
-
The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells.Curr Med Chem Anticancer Agents. 2003 May;3(3):187-99. doi: 10.2174/1568011033482440. Curr Med Chem Anticancer Agents. 2003. PMID: 12769777 Review.
-
Epigenetic regulation of osteoclast differentiation.Ann N Y Acad Sci. 2011 Dec;1240:7-13. doi: 10.1111/j.1749-6632.2011.06245.x. Ann N Y Acad Sci. 2011. PMID: 22172033 Review.
Cited by
-
Role of chromatin modulator Dpy30 in osteoclast differentiation and function.Bone. 2022 Jun;159:116379. doi: 10.1016/j.bone.2022.116379. Epub 2022 Mar 16. Bone. 2022. PMID: 35307321 Free PMC article.
-
MMP-9 drives the melanomagenic transcription program through histone H3 tail proteolysis.Oncogene. 2022 Jan;41(4):560-570. doi: 10.1038/s41388-021-02109-5. Epub 2021 Nov 16. Oncogene. 2022. PMID: 34785776
-
Regulatory Role of RNA N6-Methyladenosine Modification in Bone Biology and Osteoporosis.Front Endocrinol (Lausanne). 2020 Jan 10;10:911. doi: 10.3389/fendo.2019.00911. eCollection 2019. Front Endocrinol (Lausanne). 2020. PMID: 31998240 Free PMC article. Review.
-
Nuclear mechanosensing controls MSC osteogenic potential through HDAC epigenetic remodeling.Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21258-21266. doi: 10.1073/pnas.2006765117. Epub 2020 Aug 17. Proc Natl Acad Sci U S A. 2020. PMID: 32817542 Free PMC article.
-
Role and Regulation of Transcription Factors in Osteoclastogenesis.Int J Mol Sci. 2023 Nov 10;24(22):16175. doi: 10.3390/ijms242216175. Int J Mol Sci. 2023. PMID: 38003376 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

