Brain organoids: advances, applications and challenges
- PMID: 30992274
- PMCID: PMC6503989
- DOI: 10.1242/dev.166074
Brain organoids: advances, applications and challenges
Abstract
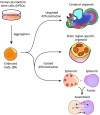
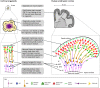
Brain organoids are self-assembled three-dimensional aggregates generated from pluripotent stem cells with cell types and cytoarchitectures that resemble the embryonic human brain. As such, they have emerged as novel model systems that can be used to investigate human brain development and disorders. Although brain organoids mimic many key features of early human brain development at molecular, cellular, structural and functional levels, some aspects of brain development, such as the formation of distinct cortical neuronal layers, gyrification, and the establishment of complex neuronal circuitry, are not fully recapitulated. Here, we summarize recent advances in the development of brain organoid methodologies and discuss their applications in disease modeling. In addition, we compare current organoid systems to the embryonic human brain, highlighting features that currently can and cannot be recapitulated, and discuss perspectives for advancing current brain organoid technologies to expand their applications.
Keywords: Brain organoids; Neuroscience; Stem cell.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Abud E. M., Ramirez R. N., Martinez E. S., Healy L. M., Nguyen C. H. H., Newman S. A., Yeromin A. V., Scarfone V. M., Marsh S. E., Fimbres C. et al. (2017). iPSC-Derived human microglia-like cells to study neurological diseases. Neuron 94, 278-293.e279. 10.1016/j.neuron.2017.03.042 - DOI - PMC - PubMed
-
- Bae B.-I., Tietjen I., Atabay K. D., Evrony G. D., Johnson M. B., Asare E., Wang P. P., Murayama A. Y., Im K., Lisgo S. N. et al. (2014). Evolutionarily dynamic alternative splicing of GPR56 regulates regional cerebral cortical patterning. Science 343, 764-768. 10.1126/science.1244392 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

