The safety and effectiveness of the Woven EndoBridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: final 12-month results of the pivotal WEB Intrasaccular Therapy (WEB-IT) Study
- PMID: 30992395
- PMCID: PMC6824604
- DOI: 10.1136/neurintsurg-2019-014815
The safety and effectiveness of the Woven EndoBridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: final 12-month results of the pivotal WEB Intrasaccular Therapy (WEB-IT) Study
Abstract
Introduction: The Woven EndoBridge Intrasaccular Therapy (WEB-IT) Study is a pivotal, prospective, single-arm, investigational device exemption study designed to evaluate the safety and effectiveness of the WEB device for the treatment of wide-neck bifurcation aneurysms.
Methods: One-hundred and fifty patients with wide-neck bifurcation aneurysms were enrolled at 21 US and six international centers. Angiograms from the index procedure, and 6-month and 1-year follow-up visits were all reviewed by a core laboratory. All adverse events were reviewed and adjudicated by a clinical events adjudicator. A data monitoring committee provided oversight during the trial to ensure subject safety.
Results: One-hundred and forty-eight patients received the WEB implant. One (0.7%) primary safety event occurred during the study-a delayed ipsilateral parenchymal hemorrhage-on postoperative day 22. No primary safety events occurred after 30 days through 1 year. At the 12-month angiographic follow-up, 77/143 patients (53.8%) had complete aneurysm occlusion. Adequate occlusion was achieved in 121/143 (84.6%) subjects.
Conclusions: The prespecified safety and effectiveness endpoints for the aneurysms studied in the WEB-IT trial were met. The results of this trial suggest that the WEB device provides an option for patients with wide-neck bifurcation aneurysms that is as effective as currently available therapies and markedly safer.
Trial registration number: NCT02191618.
Keywords: aneurysm; blood flow; coil; device; magnetic resonance angiography.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The principal investigators for the WEB-IT trial received institutional salary support for study-related activities. Investigators in the WEB-IT trial also received payment for proctoring cases within the context of the trial. ASA is a consultant for Balt, Johnson and Johnson, Leica, Medtronic, Microvention, Penumbra, Scientia, Siemens, and Stryker; receives research support from Microvention, Penumbra, and Siemens; and is a shareholder in Bendit, Cerebrotech, Endostream, Magneto, Marblehead, Neurogami, Serenity, Synchron, Triad Medical and Vascular Simulations outside the submitted work. AM is a consultant for Microvention/Sequent, Stryker Neurovascular, and Cerus outside the submitted work. ALC is a consultant for Medtronic, Microvention, and Stryker Neurovascular outside the submitted work. IS is a consultant for Medtronic and Sequent/Microvention outside the published work. IS is a consultant for Sequent/Microvention, Stryker, Medtronic, and Cerenovus outside the submitted work. DH is a consultant for Covidien/Medtronic and Microvention outside the submitted work and has received research support from Siemens. JEDA is a consultant for Medtronic, Penumbra, and Sequent outside the submitted work. LE is a consultant for Codman Neurovascular, Medtronic, MicroVention, Penumbra, Sequent, and Stryker outside the submitted work. SC is a consultant for Medtronic and Sequent/MicroVention outside the submitted work and is a stockholder in ELUM and NDI. JVB provided core laboratory services to Sequent through Oxford University during the conduct of this study and now acts as a consultant for MicroVention and Oxford Endovascular; he is a stockholder of Oxford Endovascular outside the submitted work. DF is a consultant for Balt, Marblehead, Medtronic, Stryker, Microvention, Stryker, Penumbra, and Cerenovus; receives research support from Cerenovus, Medtronic, Stryker, Siemens, Microvention, and Penumbra, and royalties from Codman; and is a stockholder for Marblehead, Neurogami, and Vascular Simulations outside the submitted work.
Figures


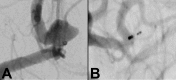
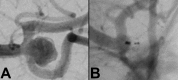

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
