Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport
- PMID: 30992489
- PMCID: PMC6468119
- DOI: 10.1038/s41598-019-42531-0
Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport
Abstract
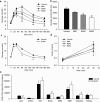
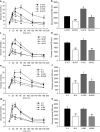
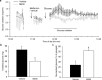
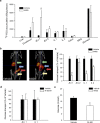
Metformin is currently the most prescribed drug for treatment of type 2 diabetes mellitus in humans. It has been well established that long-term treatment with metformin improves glucose tolerance in mice by inhibiting hepatic gluconeogenesis. Interestingly, a single dose of orally administered metformin acutely lowers blood glucose levels, however, little is known about the mechanism involved in this effect. Glucose tolerance, as assessed by the glucose tolerance test, was improved in response to prior oral metformin administration when compared to vehicle-treated mice, irrespective of whether the animals were fed either the standard or high-fat diet. Blood glucose-lowering effects of acutely administered metformin were also observed in mice lacking functional AMP-activated protein kinase, and were independent of glucagon-like-peptide-1 or N-methyl-D-aspartate receptors signaling. [18F]-FDG/PET revealed a slower intestinal transit of labeled glucose after metformin as compared to vehicle administration. Finally, metformin in a dose-dependent but indirect manner decreased glucose transport from the intestinal lumen into the blood, which was observed ex vivo as well as in vivo. Our results support the view that the inhibition of transepithelial glucose transport in the intestine is responsible for lowering blood glucose levels during an early response to oral administration of metformin.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Sterne J. Blood sugar-lowering effect of 1,1-dimethylbiguanide. Therapie. 1958;13:650–659. - PubMed
-
- Inzucchi SE, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. - DOI - PubMed
-
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet352, 854–865 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

