Synthesis of acylglycerol derivatives by mechanochemistry
- PMID: 30992730
- PMCID: PMC6444433
- DOI: 10.3762/bjoc.15.78
Synthesis of acylglycerol derivatives by mechanochemistry
Abstract
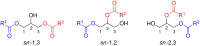
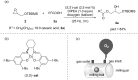
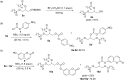
In recent times, many biologically relevant building blocks such as amino acids, peptides, saccharides, nucleotides and nucleosides, etc. have been prepared by mechanochemical synthesis. However, mechanosynthesis of lipids by ball milling techniques has remained essentially unexplored. In this work, a multistep synthetic route to access mono- and diacylglycerol derivatives by mechanochemistry has been realized, including the synthesis of diacylglycerol-coumarin conjugates.
Keywords: ball mill; coumarin; diacylglycerols; lipids; mechanochemistry.
Figures



References
-
- Garcia-Manyes S, Beedle A E M. Nat Rev Chem. 2017;1:0083. doi: 10.1038/s41570-017-0083. - DOI
LinkOut - more resources
Full Text Sources
