The unfolding of iRFP713 in a crowded milieu
- PMID: 30993043
- PMCID: PMC6459179
- DOI: 10.7717/peerj.6707
The unfolding of iRFP713 in a crowded milieu
Abstract
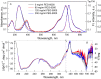
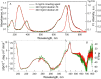
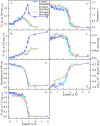
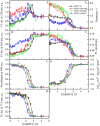
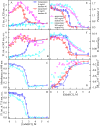
The exploring of biological processes in vitro under conditions of macromolecular crowding is a way to achieve an understanding of how these processes occur in vivo. In this work, we study the unfolding of the fluorescent probe iRFP713 in crowded environment in vitro. Previously, we showed that the unfolding of the dimeric iRFP713 is accompanied by the formation of a compact monomer and an intermediate state of the protein. In the intermediate state, the macromolecules of iRFP713 have hydrophobic clusters exposed to the surface of the protein and are prone to aggregation. Concentrated solutions of polyethylene glycol (PEG-8000), Dextran-40 and Dextran-70 with a molecular mass of 8000, 40000 and 70000 Da, respectively, were used to model the conditions for macromolecular crowding. A limited available space provided by all the crowding agents used favors to the enhanced aggregation of iRFP713 in the intermediate state at the concentration of guanidine hydrochloride (GdnHCl), at which the charge of protein surface is neutralized by the guanidine cations. This is in line with the theory of the excluded volume. In concentrated solutions of the crowding agents (240-300 mg/ml), the stabilization of the structure of iRFP713 in the intermediate state is observed. PEG-8000 also enhances the stability of iRFP713 in the monomeric compact state, whereas in concentrated solutions of Dextran-40 and Dextran-70 the resistance of the protein in the monomeric state against GdnHCl-induced unfolding decreases. The obtained data argues for the excluded volume effect being not the only factor that contributes the behavior of biological molecules in a crowded milieu. Crowding agents do not affect the structure of the native dimer of iRFP713, which excludes the direct interactions between the target protein and the crowding agents. PEGs of different molecular mass and Dextran-40/Dextran-70 are known to influence the solvent properties of water. The solvent dipolarity/polarizability and basicity/acidity in aqueous solutions of these crowding agents vary in different ways. The change of the solvent properties in aqueous solutions of crowding agents might impact the functioning of a target protein.
Keywords: Crowded milieu; Excluded volume effect; Protein aggregation; Protein unfolding; Solvent properties; iRFP713.
Conflict of interest statement
Konstantin K. Turoverov and Irina M. Kuznetsova are Academic Editors for PeerJ.
Figures




Similar articles
-
The Pathways of the iRFP713 Unfolding Induced by Different Denaturants.Int J Mol Sci. 2018 Sep 15;19(9):2776. doi: 10.3390/ijms19092776. Int J Mol Sci. 2018. PMID: 30223568 Free PMC article.
-
Structure and Conformational Properties of d-Glucose/d-Galactose-Binding Protein in Crowded Milieu.Molecules. 2017 Feb 6;22(2):244. doi: 10.3390/molecules22020244. Molecules. 2017. PMID: 28178192 Free PMC article.
-
Role of solvent properties of aqueous media in macromolecular crowding effects.J Biomol Struct Dyn. 2016;34(1):92-103. doi: 10.1080/07391102.2015.1011235. Epub 2015 Feb 26. J Biomol Struct Dyn. 2016. PMID: 25616385
-
The macromolecular crowding effect--from in vitro into the cell.Biol Chem. 2016 Jan;397(1):37-44. doi: 10.1515/hsz-2015-0161. Biol Chem. 2016. PMID: 26351910 Review.
-
Beyond the excluded volume effects: mechanistic complexity of the crowded milieu.Molecules. 2015 Jan 14;20(1):1377-409. doi: 10.3390/molecules20011377. Molecules. 2015. PMID: 25594347 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

