Calcium-looping reforming of methane realizes in situ CO2 utilization with improved energy efficiency
- PMID: 30993203
- PMCID: PMC6461455
- DOI: 10.1126/sciadv.aav5077
Calcium-looping reforming of methane realizes in situ CO2 utilization with improved energy efficiency
Abstract
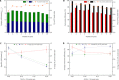
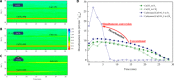
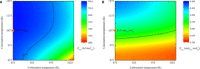
Closing the anthropogenic carbon cycle is one important strategy to combat climate change, and requires the chemistry to effectively combine CO2 capture with its conversion. Here, we propose a novel in situ CO2 utilization concept, calcium-looping reforming of methane, to realize the capture and conversion of CO2 in one integrated chemical process. This process couples the calcium-looping CO2 capture and the CH4 dry reforming reactions in the CaO-Ni bifunctional sorbent-catalyst, where the CO2 captured by CaO is reduced in situ by CH4 to CO, a reaction catalyzed by catalyzed by the adjacent metallic Ni. The process coupling scheme exhibits excellent decarbonation kinetics by exploiting Le Chatelier's principle to shift reaction equilibrium through continuous conversion of CO2, and results in an energy consumption 22% lower than that of conventional CH4 dry reforming for CO2 utilization. The proposed CO2 utilization concept offers a promising option to recycle carbon directly at large CO2 stationary sources in an energy-efficient manner.
Figures



References
-
- Rogelj J., den Elzen M., Höhne N., Fransen T., Fekete H., Winkler H., Schaeffer R., Sha F., Riahi K., Meinshausen M., Paris agreement climate proposals need a boost to keep warming well below 2°C. Nature 534, 631–639 (2016). - PubMed
-
- International Energy Agency, Energy Technology Perspectives 2016: Towards Sustainable Urban Energy Systems (International Energy Agency, 2016).
-
- International Energy Agency, Energy Technology Perspectives 2012: Pathways to a Clean Energy System (International Energy Agency, 2012); www.iea.org/publications/freepublications/publication/ETP2012_free.pdf.
-
- Otto A., Grube T., Schiebahn S., Stolten D., Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 8, 3283–3297 (2015).
-
- Abanades J. C., Rubin E. S., Mazzotti M., Herzog H. J., On the climate change mitigation potential of CO2 conversion to fuels. Energy Environ. Sci. 10, 2491–2499 (2017).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

