Mucinous rectal cancer: concepts and imaging challenges
- PMID: 30993392
- PMCID: PMC7357860
- DOI: 10.1007/s00261-019-02019-x
Mucinous rectal cancer: concepts and imaging challenges
Abstract
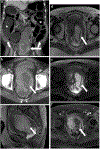
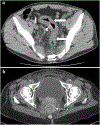
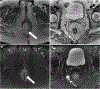
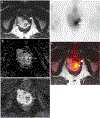
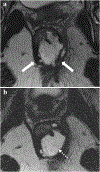
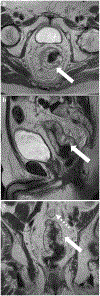
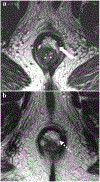
Rectal adenocarcinoma with mucinous components is an uncommon type of rectal cancer with two distinct histologic subtypes: mucinous adenocarcinoma and signet-ring cell carcinoma. Mucin can also be identified as pattern of response after neoadjuvant treatment. On imaging modalities, mucin typically demonstrates high signal intensity on T2-weighted images, low attenuation on computed tomography, and may be negative on 18-fluorodeoxyglucose positron emission tomography. After neoadjuvant CRT, cellular and acellular mucin share similar imaging features, and differentiating them is currently the main challenge faced by radiologists. Radiologists should be aware of pros, cons, and limitations of each imaging modality in the primary staging and restaging to avoid misinterpretation of the radiological findings.
Keywords: Computed tomography; Magnetic resonance imaging; Mucin; Positron emission tomography; Rectal neoplasms.
Conflict of interest statement
Figures

References
-
- Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System: World Health Organization, International Agency for Research on Cancer, 2010.
-
- Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, Slotta-Huspenina J, Käser SA, Michalski CW, Janssen KP, Friess H, Rosenberg R, Bader FG. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 2013;258(5):775–782; discussion 782–773. doi: 10.1097/SLA.0b013e3182a69f7e - DOI - PMC - PubMed
-
- Xie L, Villeneuve PJ, Shaw A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int J Oncol 2009;34(4):1109–1115. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

