A single-arm phase II trial of weekly nanoparticle albumin-bound paclitaxel (nab-paclitaxel) monotherapy after standard of chemotherapy for previously treated advanced non-small cell lung cancer
- PMID: 30993397
- PMCID: PMC6647220
- DOI: 10.1007/s00280-019-03843-0
A single-arm phase II trial of weekly nanoparticle albumin-bound paclitaxel (nab-paclitaxel) monotherapy after standard of chemotherapy for previously treated advanced non-small cell lung cancer
Abstract
Background: Few studies have investigated the clinical efficacy of third- and later-line of chemotherapy after standard chemotherapy for previously treated advanced non-small cell lung cancer (NSCLC). We prospectively evaluated the efficacy and safety of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) following standard chemotherapies for previously treated advanced NSCLC.
Methods: The eligible patients having adequate organ functions with performance status 0-2 were enrolled after completing standard chemotherapy. They received weekly nab-paclitaxel 100 mg/m2 intravenously on days 1, 8, and 15 every 3 weeks. The primary end point was objective response rate (ORR). Median progression-free survival (PFS), overall survival (OS), and adverse events (AEs) were evaluated as secondary end points.
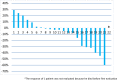
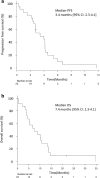
Results: This trial was discontinued because of late accrual. Twenty two patients were enrolled from April 2013 and February 2019. The total ORR was 22.7% [95% CI 7.8-45.4] and disease control rate (DCR) was 81.8% [95% CI 59.7-94.8]. Median PFS was 3.4 months [95% CI 2.3-4.1] and median OS was 7.4 months [95% CI 4.2-10.7]. Median follow-up interval was 6.7 months hematological AEs of Grade 3/4 included anemia (18%), leukopenia (18%), and neutropenia (32%), while the most frequent nonhematological AEs were fatigue (50%) and peripheral neuropathy (36.4%). Severe AEs related to treatment were observed in only one patient.
Conclusion: Nab-paclitaxel may be a safe and effective later-line chemotherapeutic option for previously treated advanced NSCLC after standard of chemotherapies based on other trials.
Keywords: Advanced non-small cell lung cancer; Chemotherapy; Later line setting; Nanoparticle albumin-bound paclitaxel.
Conflict of interest statement
YH and YO have received honoraria from TAIHO PHARMACEUTICAL. The other authors declare no conflicts of interest associated with this manuscript.
Figures
References
-
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. - DOI - PubMed
-
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, Rodriguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous