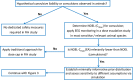
A Framework Proposal to Follow-Up on Preclinical Convulsive Signals of a New Molecular Entity in First-in-Human Studies Using Electroencephalographic Monitoring
- PMID: 30993670
- PMCID: PMC6851537
- DOI: 10.1002/cpt.1455
A Framework Proposal to Follow-Up on Preclinical Convulsive Signals of a New Molecular Entity in First-in-Human Studies Using Electroencephalographic Monitoring
Abstract
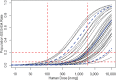
Traditionally, in dose-escalating first-in-human (FiH) studies, a dose cap with a 10-fold safety margin to the no observed effect level in animals is implemented if convulsive events are observed in animals. However, the convulsive risk seen in animals does not generally translate to humans. Several lines of evidence are summarized indicating that in a dose-escalating setting, electroencephalographic epileptiform abnormalities occur at lower doses than clinical convulsive events. Therefore, we propose to consider the occurrence of epileptiform abnormalities in toxicology studies as premonitory signals for convulsions in dose-escalating FiH studies. Compared with the traditional dose-cap approach, this may allow the exploration of higher doses in FiH and, subsequently, phase II studies without compromising human safety. Similarly, the presence or absence of electroencephalographic epileptiform abnormalities may also aid the assessment of proconvulsive risk in situations of increased perpetrator burden as potentially present in pharmacokinetic and/or pharmacodynamic drug-drug interactions.
© 2019 F. Hoffmann-La Roche AG. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.A., T.D., A.R., E.H., R.P., and C.W. are employees of F. Hoffmann‐La Roche AG. R.D. and K.K. provided consultancy to F. Hoffmann‐La Roche AG. L.D. is a previous employee of F. Hoffmann‐La Roche AG.
Figures

Similar articles
-
Exploratory EEG studies for the assessment of neurological liabilities in conscious dogs in early drug development.J Pharmacol Toxicol Methods. 2019 Jul-Aug;98:106581. doi: 10.1016/j.vascn.2019.106581. Epub 2019 May 15. J Pharmacol Toxicol Methods. 2019. PMID: 31102738
-
A brief survey of first-in-human studies.J Clin Pharmacol. 2011 Jul;51(7):988-93. doi: 10.1177/0091270010377631. Epub 2010 Jul 29. J Clin Pharmacol. 2011. PMID: 20671293
-
Assessment of seizure risk in pre-clinical studies: Strengths and limitations of the electroencephalogram (EEG).J Pharmacol Toxicol Methods. 2015 Sep-Oct;75:135-42. doi: 10.1016/j.vascn.2015.04.001. Epub 2015 Apr 16. J Pharmacol Toxicol Methods. 2015. PMID: 25891491 No abstract available.
-
On setting the first dose in man: quantitating biotherapeutic drug-target binding through pharmacokinetic and pharmacodynamic models.Basic Clin Pharmacol Toxicol. 2010 Mar;106(3):195-209. doi: 10.1111/j.1742-7843.2009.00513.x. Epub 2009 Dec 29. Basic Clin Pharmacol Toxicol. 2010. PMID: 20050847 Review.
-
An FDA oncology analysis of CD3 bispecific constructs and first-in-human dose selection.Regul Toxicol Pharmacol. 2017 Nov;90:144-152. doi: 10.1016/j.yrtph.2017.09.001. Epub 2017 Sep 5. Regul Toxicol Pharmacol. 2017. PMID: 28887049 Review.
References
-
- Clark, M. & Steger‐Hartmann, T. A big data approach to the concordance of the toxicity of pharmaceuticals in animals and humans. Reg. Toxicol. Pharmacol. 96, 94–105 (2018). - PubMed
-
- Bass, A. et al Origins, practices and future of safety pharmacology. J. Pharmacol. Toxicol. Meth. 49, 145–151 (2004). - PubMed
-
- Kinter, L.B. et al Major organ systems toxicology: an integrated approach to pharmacodynamic safety assessment studies in animals In Comprehensive Toxicology: Vol. 2. Toxicology Testing and Evaluation (eds. Williams P.D. and Hottendorf G.H. (Series), Sipes I.G., McQueen C.A. and Gandolfi A.J. (Vol.)) 155–168 (Elsevier, New York, 1997).
-
- Bassett, L. et al Telemetry video‐electroencephalography (EEG) in rats, dogs and non‐human primates: methods in follow‐up safety pharmacology seizure liability assessments. J. Pharmacol. Toxicol. Meth. 70, 230–240 (2014). - PubMed
-
- Authier, S. et al Safety pharmacology investigations on the nervous system: an industry survey. J. Pharmacol. Toxicol. Meth. 81, 37–46 (2016). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

