Salidroside ameliorates Adriamycin nephropathy in mice by inhibiting β-catenin activity
- PMID: 30993911
- PMCID: PMC6533469
- DOI: 10.1111/jcmm.14340
Salidroside ameliorates Adriamycin nephropathy in mice by inhibiting β-catenin activity
Abstract
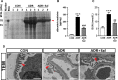
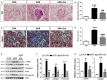
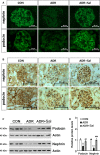
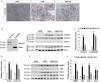
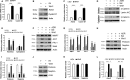
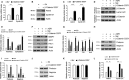
Salidroside is a major phenylethanoid glycoside in Rhodiola rosea L., a traditional Chinese medicine, with multiple biological activities. It has been shown that salidroside possesses protective effects for alleviating diabetic renal dysfunction, contrast-induced-nephropathy and other kidney diseases. However, the involved molecular mechanism was still not understood well. Herein, we examined the protective effects of salidroside in mice with Adriamycin (ADR)-induced nephropathy and the underlying molecular mechanism. The results showed that salidroside treatment ameliorates proteinuria; improves expressions of nephrin and podocin; and reduces kidney fibrosis and glomerulosclerosis induced by ADR. Mechanistically, ADR induces a robust accumulation of β-catenin in the nucleus and stimulates its downstream target gene expression. The application of salidroside largely abolishes the nuclear translocation of β-catenin and thus inhibits its activity. Furthermore, the activation of β-catenin almost completely counteracts the protective roles of salidroside in ADR-injured podocytes. Taken together, our data indicate that salidroside ameliorates proteinuria, renal fibrosis and podocyte injury in ADR nephropathy, which may rely on inhibition of β-catenin signalling pathway.
Keywords: Adriamycin nephropathy; podocytes; salidroside; β-catenin.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury.J Am Soc Nephrol. 2011 Jan;22(1):90-103. doi: 10.1681/ASN.2009121236. Epub 2010 Oct 28. J Am Soc Nephrol. 2011. PMID: 21030600 Free PMC article.
-
Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice.Phytomedicine. 2019 Feb 15;54:240-247. doi: 10.1016/j.phymed.2018.10.031. Epub 2018 Oct 25. Phytomedicine. 2019. PMID: 30668374
-
Protective role of cyclosporine A and minocycline on mitochondrial disequilibrium-related podocyte injury and proteinuria occurrence induced by adriamycin.Nephrol Dial Transplant. 2015 Jun;30(6):957-69. doi: 10.1093/ndt/gfv015. Epub 2015 Feb 1. Nephrol Dial Transplant. 2015. PMID: 25646018
-
Pharmacological functions of salidroside in renal diseases: facts and perspectives.Front Pharmacol. 2024 Jan 8;14:1309598. doi: 10.3389/fphar.2023.1309598. eCollection 2023. Front Pharmacol. 2024. PMID: 38259279 Free PMC article. Review.
-
Salidroside: A review of its recent advances in synthetic pathways and pharmacological properties.Chem Biol Interact. 2021 Apr 25;339:109268. doi: 10.1016/j.cbi.2020.109268. Epub 2021 Feb 20. Chem Biol Interact. 2021. PMID: 33617801 Review.
Cited by
-
Recent Advances in Traditional Chinese Medicine for Treatment of Podocyte Injury.Front Pharmacol. 2022 Feb 25;13:816025. doi: 10.3389/fphar.2022.816025. eCollection 2022. Front Pharmacol. 2022. PMID: 35281899 Free PMC article. Review.
-
IgA1 deposition may induce NLRP3 expression and macrophage transdifferentiation of podocyte in IgA nephropathy.J Transl Med. 2019 Dec 3;17(1):406. doi: 10.1186/s12967-019-02157-2. J Transl Med. 2019. PMID: 31796125 Free PMC article.
-
Natural products in traditional Chinese medicine for renal fibrosis: a comprehensive review.Front Pharmacol. 2025 Apr 16;16:1560567. doi: 10.3389/fphar.2025.1560567. eCollection 2025. Front Pharmacol. 2025. PMID: 40308781 Free PMC article. Review.
-
Natural products: potential drugs for the treatment of renal fibrosis.Chin Med. 2022 Aug 17;17(1):98. doi: 10.1186/s13020-022-00646-z. Chin Med. 2022. PMID: 35978370 Free PMC article. Review.
-
Natural products for kidney disease treatment: Focus on targeting mitochondrial dysfunction.Front Pharmacol. 2023 Mar 15;14:1142001. doi: 10.3389/fphar.2023.1142001. eCollection 2023. Front Pharmacol. 2023. PMID: 37007023 Free PMC article. Review.
References
-
- Thomas MC. Pathogenesis and progression of proteinuria. Contrib Nephrol. 2011;170:48‐56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

