Evaluation of Renal Pathophysiological Processes Induced by an Iodinated Contrast Agent in a Diabetic Rabbit Model Using Intravoxel Incoherent Motion and Blood Oxygenation Level-Dependent Magnetic Resonance Imaging
- PMID: 30993934
- PMCID: PMC6470079
- DOI: 10.3348/kjr.2018.0757
Evaluation of Renal Pathophysiological Processes Induced by an Iodinated Contrast Agent in a Diabetic Rabbit Model Using Intravoxel Incoherent Motion and Blood Oxygenation Level-Dependent Magnetic Resonance Imaging
Abstract
Objective: To examine the potential of intravoxel incoherent motion (IVIM) and blood oxygen level-dependent (BOLD) magnetic resonance imaging for detecting renal changes after iodinated contrast-induced acute kidney injury (CI-AKI) development in a diabetic rabbit model.
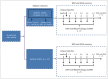
Materials and methods: Sixty-two rabbits were randomized into 2 groups: diabetic rabbits with the contrast agent (DCA) and healthy rabbits with the contrast agent (NCA). In each group, 6 rabbits underwent IVIM and BOLD imaging at 1 hour, 1 day, 2 days, 3 days, and 4 days after an iohexol injection while 5 rabbits were selected to undergo blood and histological examinations at these specific time points. Iohexol was administrated at a dose of 2.5 g I/kg of body weight. Further, the apparent transverse relaxation rate (R2*), average pure molecular diffusion coefficient (D), pseudo-diffusion coefficient (D*), and perfusion fraction (f) were calculated.
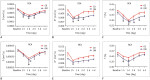
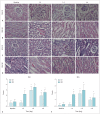
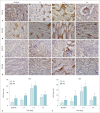
Results: The D and f values of the renal cortex (CO) and outer medulla (OM) were significantly decreased compared to baseline values in the 2 groups 1 day after the iohexol injection (p < 0.05). A marked reduction in the D* values for both the CO and OM was also observed after 1 hour in each group (p < 0.05). In the OM, a persistent elevation of the R2* was detected for 4 days in the DCA group (p < 0.05). Histopathological changes were prominent, and the pathological features of CI-AKI aggravated in the DCA group until day 4. The D, f, and R2* values significantly correlated with the histological damage scores, hypoxia-inducible transcription factor-1α expression scores, and serum creatinine levels.
Conclusion: A combination of IVIM and BOLD imaging may serve as a noninvasive method for detecting and monitoring CI-AKI in the early stages in the diabetic kidney.
Keywords: Hypoxia-inducible transcription factor-1α; Medullary hypoxia; Renal vascular dysfunction; Vascular endothelial growth factor.
Copyright © 2019 The Korean Society of Radiology.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures





References
-
- McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428. - PubMed
-
- Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR) Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. - PubMed
-
- Chalikias G, Drosos I, Tziakas DN. Contrast-induced acute kidney injury: an update. Cardiovasc Drugs Ther. 2016;30:215–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

