Arhgef1 Plays a Vital Role in Platelet Function and Thrombogenesis
- PMID: 30994039
- PMCID: PMC6512111
- DOI: 10.1161/JAHA.118.011712
Arhgef1 Plays a Vital Role in Platelet Function and Thrombogenesis
Abstract
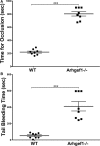
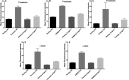
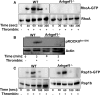
Background Platelets are the cellular mediators of hemostasis and thrombosis, and their function is regulated by a number of molecular mediators, such as small GTP ases. These small GTP ases are themselves regulated by guanine nucleotide exchange factors such as Arhgefs, several of which are found in platelets, including the highly expressed Arhgef1. However, the role of Arhgef1 in platelets has not yet been investigated. Methods and Results We employed mice with genetic deletion of Arhgef1 (ie, Arhgef1-/-) and investigated their platelet phenotype by employing a host of in vivo and in vitro platelet assays. Our results indicate that Arhgef1-/- mice had prolonged carotid artery occlusion and tail bleeding times. Moreover, platelets from these mice exhibited defective aggregation, dense and α granule secretion, α II bβ3 integrin activation, clot retraction and spreading, in comparison to their wild-type littermates. Finally, we also found that the mechanism by which Arhgef1 regulates platelets is mediated in part by a defect in the activation of the RhoA-Rho-associated kinase axis, but not Rap1b. Conclusions Our data demonstrate, for the first time, that Arhgef1 plays a critical role in platelet function, in vitro and in vivo.
Keywords: Arhgef1; cardiovascular diseases; platelets; small GTPases; thrombosis.
Figures



Similar articles
-
ARHGEF10 knockout inhibits platelet aggregation and protects mice from thrombus formation.J Thromb Haemost. 2017 Oct;15(10):2053-2064. doi: 10.1111/jth.13799. Epub 2017 Sep 18. J Thromb Haemost. 2017. PMID: 28799234
-
Leukemia-associated Rho guanine-nucleotide exchange factor is not critical for RhoA regulation, yet is important for platelet activation and thrombosis in mice.J Thromb Haemost. 2015 Nov;13(11):2102-7. doi: 10.1111/jth.13129. Epub 2015 Oct 20. J Thromb Haemost. 2015. PMID: 26334261 Free PMC article.
-
Reelin Amplifies Glycoprotein VI Activation and AlphaIIb Beta3 Integrin Outside-In Signaling via PLC Gamma 2 and Rho GTPases.Arterioscler Thromb Vasc Biol. 2020 Oct;40(10):2391-2403. doi: 10.1161/ATVBAHA.120.314902. Epub 2020 Aug 13. Arterioscler Thromb Vasc Biol. 2020. PMID: 32787521
-
Platelet-based coagulation: different populations, different functions.J Thromb Haemost. 2013 Jan;11(1):2-16. doi: 10.1111/jth.12045. J Thromb Haemost. 2013. PMID: 23106920 Review.
-
MicroRNA Biomarkers and Platelet Reactivity: The Clot Thickens.Circ Res. 2017 Jan 20;120(2):418-435. doi: 10.1161/CIRCRESAHA.116.309303. Circ Res. 2017. PMID: 28104774 Review.
Cited by
-
The JUUL E-Cigarette Elevates the Risk of Thrombosis and Potentiates Platelet Activation.J Cardiovasc Pharmacol Ther. 2020 Nov;25(6):578-586. doi: 10.1177/1074248420941681. Epub 2020 Jul 21. J Cardiovasc Pharmacol Ther. 2020. PMID: 32691614 Free PMC article.
-
Short-Term Exposure to Waterpipe/Hookah Smoke Triggers a Hyperactive Platelet Activation State and Increases the Risk of Thrombogenesis.Arterioscler Thromb Vasc Biol. 2020 Feb;40(2):335-349. doi: 10.1161/ATVBAHA.119.313435. Epub 2020 Jan 16. Arterioscler Thromb Vasc Biol. 2020. PMID: 31941383 Free PMC article.
-
Turning Platelets Off and On: Role of RhoGAPs and RhoGEFs in Platelet Activity.Front Cardiovasc Med. 2022 Jan 6;8:820945. doi: 10.3389/fcvm.2021.820945. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35071371 Free PMC article. Review.
References
-
- Aslan JE, Itakura A, Gertz JM, McCarty OJT. Platelet shape change and spreading In: Gibbins JM, Mahaut‐Smith MP, eds. Platelets and Megakaryocytes. Vol 3, Additional Protocols and Perspectives. New York, NY: Springer New York; 2012:91–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

