mTORC1 is required for expression of LRPPRC and cytochrome- c oxidase but not HIF-1α in Leigh syndrome French Canadian type patient fibroblasts
- PMID: 30995105
- PMCID: PMC6689754
- DOI: 10.1152/ajpcell.00160.2017
mTORC1 is required for expression of LRPPRC and cytochrome- c oxidase but not HIF-1α in Leigh syndrome French Canadian type patient fibroblasts
Abstract
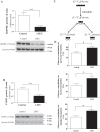
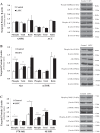
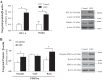
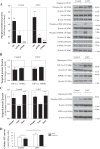
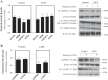
Leigh syndrome French Canadian type (LSFC) is a mitochondrial disease caused by mutations in the leucine-rich pentatricopeptide repeat-containing (LRPPRC) gene leading to a reduction of cytochrome-c oxidase (COX) expression reaching 50% in skin fibroblasts. We have shown that under basal conditions, LSFC and control cells display similar ATP levels. We hypothesized that this occurs through upregulation of mechanistic target of rapamycin (mTOR)-mediated metabolic reprogramming. Our results showed that compared with controls, LSFC cells exhibited an upregulation of the mTOR complex 1 (mTORC1)/p70 ribosomal S6 kinase pathway and higher levels of hypoxia-inducible factor 1α (HIF-1α) and its downstream target pyruvate dehydrogenase kinase 1 (PDHK1), a regulator of mitochondrial pyruvate dehydrogenase 1 (PDH1). Consistent with these signaling alterations, LSFC cells displayed a 40-61% increase in [U-13C6]glucose contribution to pyruvate, lactate, and alanine formation, as well as higher levels of the phosphorylated and inactive form of PDH1-α. Interestingly, inhibition of mTOR with rapamycin did not alter HIF-1α or PDHK1 protein levels in LSFC fibroblasts. However, this treatment increased PDH1-α phosphorylation in control and LSFC cells and reduced ATP levels in control cells. Rapamycin also decreased LRPPRC expression by 41 and 11% in LSFC and control cells, respectively, and selectively reduced COX subunit IV expression in LSFC fibroblasts. Taken together, our data demonstrate the importance of mTORC1, independent of the HIF-1α/PDHK1 axis, in maintaining LRPPRC and COX expression in LSFC cells.
Keywords: COX; LRPPRC; LSFC; mTORC1; mitochondrial diseases; rapamycin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Expression signature of the Leigh syndrome French-Canadian type.Mol Genet Metab Rep. 2022 Feb 5;30:100847. doi: 10.1016/j.ymgmr.2022.100847. eCollection 2022 Mar. Mol Genet Metab Rep. 2022. PMID: 35242578 Free PMC article.
-
Low-concentration methylene blue maintains energy production and strongly improves survival of Leigh syndrome French Canadian skin fibroblasts.J Pharm Pharm Sci. 2011;14(3):438-49. doi: 10.18433/j3m01x. J Pharm Pharm Sci. 2011. PMID: 22202226
-
The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA.Biochem J. 2004 Aug 15;382(Pt 1):331-6. doi: 10.1042/BJ20040469. Biochem J. 2004. PMID: 15139850 Free PMC article.
-
Biochemical defects and genetic abnormalities in cytochrome c oxidase of patients with Leigh syndrome.Biofactors. 1998;7(3):273-6. doi: 10.1002/biof.5520070326. Biofactors. 1998. PMID: 9568266 Review. No abstract available.
-
Human cytochrome oxidase deficiency.Pediatr Res. 2000 Nov;48(5):581-5. doi: 10.1203/00006450-200011000-00004. Pediatr Res. 2000. PMID: 11044474 Review.
Cited by
-
Mechanisms of mitochondrial respiratory adaptation.Nat Rev Mol Cell Biol. 2022 Dec;23(12):817-835. doi: 10.1038/s41580-022-00506-6. Epub 2022 Jul 8. Nat Rev Mol Cell Biol. 2022. PMID: 35804199 Free PMC article. Review.
-
Mitochondrial leak metabolism induces the Spemann-Mangold Organizer via Hif-1α in Xenopus.Dev Cell. 2023 Nov 20;58(22):2597-2613.e4. doi: 10.1016/j.devcel.2023.08.015. Epub 2023 Sep 5. Dev Cell. 2023. PMID: 37673063 Free PMC article.
-
Expression signature of the Leigh syndrome French-Canadian type.Mol Genet Metab Rep. 2022 Feb 5;30:100847. doi: 10.1016/j.ymgmr.2022.100847. eCollection 2022 Mar. Mol Genet Metab Rep. 2022. PMID: 35242578 Free PMC article.
-
Human induced pluripotent stem cells (hiPSCs) derived cells reflect tissue specificity found in patients with Leigh syndrome French Canadian variant (LSFC).Front Genet. 2024 Apr 19;15:1375467. doi: 10.3389/fgene.2024.1375467. eCollection 2024. Front Genet. 2024. PMID: 38706791 Free PMC article.
-
Novel LRPPRC compound heterozygous mutation in a child with early-onset Leigh syndrome French-Canadian type: case report of an Italian patient.Ital J Pediatr. 2020 Sep 24;46(1):140. doi: 10.1186/s13052-020-00903-7. Ital J Pediatr. 2020. PMID: 32972427 Free PMC article.
References
-
- Burelle Y, Bemeur C, Rivard ME, Thompson Legault J, Boucher G, Morin C, Coderre L, Des Rosiers C; LSFC Consortium . Mitochondrial vulnerability and increased susceptibility to nutrient-induced cytotoxicity in fibroblasts from Leigh syndrome French Canadian patients. PLoS One 10: e0120767, 2015. doi:10.1371/journal.pone.0120767. - DOI - PMC - PubMed
-
- Cerniglia GJ, Dey S, Gallagher-Colombo SM, Daurio NA, Tuttle S, Busch TM, Lin A, Sun R, Esipova TV, Vinogradov SA, Denko N, Koumenis C, Maity A. The PI3K/Akt pathway regulates oxygen metabolism via pyruvate dehydrogenase (PDH)-E1α phosphorylation. Mol Cancer Ther 14: 1928–1938, 2015. doi:10.1158/1535-7163.MCT-14-0888. - DOI - PMC - PubMed
-
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: 1250684, 2014. [Erratum in Science 346: aaa1503, 2014.] doi:10.1126/science.1250684. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

