Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia
- PMID: 30995176
- PMCID: PMC10448866
- DOI: 10.1200/JCO.18.01460
Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia
Abstract
Purpose: A randomized, double-blind, phase III study of idelalisib (IDELA) plus rituximab versus placebo plus rituximab in patients with relapsed chronic lymphocytic leukemia (CLL) was terminated early because of superior efficacy of the IDELA-plus-rituximab (IDELA/R) arm. Patients in either arm could then enroll in an extension study to receive IDELA monotherapy. Here, we report the long-term efficacy and safety data for IDELA-treated patients across the primary and extension studies.
Patients and methods: Patients were randomly assigned to receive rituximab in combination with either IDELA 150 mg twice daily (IDELA/R; n = 110) or placebo (placebo/R; n = 110). Key end points were progression-free survival (PFS), overall response rate (ORR), overall survival (OS), and safety.
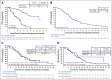
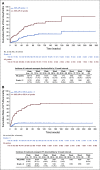
Results: The long-term efficacy and safety of treatment with IDELA was assessed in 110 patients who received at least one dose of IDELA in the primary study, 75 of whom enrolled in the extension study. The IDELA/R-to-IDELA group had a median PFS of 20.3 months (95% CI, 17.3 to 26.3 months) after a median follow-up time of 18 months (range, 0.3 to 67.6 months). The ORR was 85.5% (94 of 110 patients; n = 1 complete response). The median OS was 40.6 months (95% CI, 28.5 to 57.3 months) and 34.6 months (95% CI, 16.0 months to not reached) for patients randomly assigned to the IDELA/R and placebo/R groups, respectively. Prolonged exposure to IDELA increased the incidence of all-grade, grade 2, and grade 3 or greater diarrhea (46.4%, 17.3%, and 16.4%, respectively), all-grade and grade 3 or greater colitis (10.9% and 8.2%, respectively) and all-grade and grade 3 or greater pneumonitis (10.0% and 6.4%, respectively) but did not increase the incidence of elevated hepatic aminotransferases.
Conclusion: IDELA improved PFS and OS compared with rituximab alone in patients with relapsed CLL. Long-term IDELA was effective and had an expected safety profile. No new IDELA-related adverse events were identified with longer exposure.
Conflict of interest statement
Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Jeff P. Sharman
Steven E. Coutre
Richard R. Furman
Bruce D. Cheson
John M. Pagel
Peter Hillmen
Jacqueline C. Barrientos
Andrew D. Zelenetz
Bertrand Coiffier
Thomas J. Kipps
Ian W. Flinn
Paolo Ghia
Herbert Eradat
Nicole Lamanna
Bertrand Coiffier
Andrew R. Pettitt
Shuo Ma
Eugen Tausch
Paula Cramer
Julie Huang
Siddhartha Mitra
Michael Hallek
Susan M. O’Brien
Stephan Stilgenbauer
No other potential conflicts of interest were reported.
Figures


Comment in
-
Idelalisib and rituximab for chronic lymphocytic leukaemia.Lancet Oncol. 2019 Jun;20(6):e294. doi: 10.1016/S1470-2045(19)30250-5. Epub 2019 Apr 27. Lancet Oncol. 2019. PMID: 31036467 No abstract available.
References
-
- SEER :Stat fact sheets: Chronic lymphocytic leukemia. https://seer.cancer.gov/statfacts/html/clyl.html
-
- Shanafelt T:Treatment of older patients with chronic lymphocytic leukemia: Key questions and current answers.Hematology (Am Soc Hematol Educ Program) 2013:158-167,2013 - PubMed
-
- Smolej L:Therapy of elderly/comorbid patients with chronic lymphocytic leukemia.Curr Pharm Des 18:3399-3405,2012 - PubMed
-
- Sharman JP,Mato A,Kay NE,et al. : Demographics by age group and line of therapy in Chronic lymphocytic leukemia patients treated in US practices from the Connect CLL registry. Blood 124:3338, 2014
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

