Competence of non-human primates to transmit Leishmania infantum to the invertebrate vector Lutzomyia longipalpis
- PMID: 30995227
- PMCID: PMC6488095
- DOI: 10.1371/journal.pntd.0007313
Competence of non-human primates to transmit Leishmania infantum to the invertebrate vector Lutzomyia longipalpis
Abstract
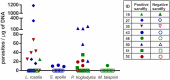
Leishmaniasis is a zoonotic disease of worldwide relevance. Visceral leishmaniasis is endemic in Brazil, where it is caused by Leishmania infantum with Lutzomyia longipalpis being the most important invertebrate vector. Non-human primates are susceptible to L. infantum infection. However, little is known about the role of these species as reservoirs. The aim of this study was to evaluate the transmissibility potential of visceral leishmaniasis by non-human primates through xenodiagnosis using the phlebotomine Lu. longipalpis as well as to identify phlebotomine species prevalent in the area where the primates were kept in captivity, and assess infection by Leishmania in captured phlebotomine specimens. Fifty two non-human primates kept in captivity in an endemic area for leishmaniasis were subjected to xenodiagnosis. All primates were serologically tested for detection of anti-Leishmania antibodies. Additionally, an anti-Lu. longipalpis saliva ELISA was performed. Sand flies fed on all animals were tested by qPCR to identify and quantify L. infantum promastigotes. Eight of the 52 non-human primates were positive by xenodiagnosis, including three Pan troglodytes, three Leontopithecus rosalia, one Sapajus apella, and one Miopithecus talapoin, with estimated numbers of promastigotes ranging from 5.67 to 1,181.93 per μg of DNA. Positive animals had higher levels of IgG anti-Lu. longipalpis saliva when compared to negative animals, prior to xenodiagnosis. Captive non-human primates are capable of infecting Lu. longipalpis with L. infantum. Our findings also demonstrate the relevance of non-human primates as sentinels to zoonotic diseases. Several phlebotomine species, including Lu. longipalpis, have been identified in the area where the primates were maintained, but only one pool of Lutzomyia lenti was infected with L. infantum. This study has implications for public health strategies and conservation medicine.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Transmissibility of Leishmania infantum from maned wolves (Chrysocyon brachyurus) and bush dogs (Speothos venaticus) to Lutzomyia longipalpis.Vet Parasitol. 2015 Sep 15;212(3-4):86-91. doi: 10.1016/j.vetpar.2015.08.024. Epub 2015 Aug 28. Vet Parasitol. 2015. PMID: 26342623
-
Epidemiological aspects of vector, parasite, and domestic reservoir in areas of recent transmission and no reported human cases of visceral leishmaniasis in Brazil.Acta Trop. 2015 Aug;148:128-36. doi: 10.1016/j.actatropica.2015.04.002. Epub 2015 Apr 14. Acta Trop. 2015. PMID: 25882769
-
Natural infection by Leishmania infantum in the Lutzomyia longipalpis population of an endemic coastal area to visceral leishmaniasis in Brazil is not associated with bioclimatic factors.PLoS Negl Trop Dis. 2019 Aug 26;13(8):e0007626. doi: 10.1371/journal.pntd.0007626. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31449534 Free PMC article.
-
Lutzomyia longipalpis (Sand Fly).Trends Parasitol. 2020 Sep;36(9):796-797. doi: 10.1016/j.pt.2020.05.007. Epub 2020 May 26. Trends Parasitol. 2020. PMID: 32467046 Review. No abstract available.
-
Lutzomyia longipalpis, Gone with the Wind and Other Variables.Neotrop Entomol. 2021 Apr;50(2):161-171. doi: 10.1007/s13744-020-00811-9. Epub 2020 Aug 25. Neotrop Entomol. 2021. PMID: 32840741 Review.
Cited by
-
Canine leishmaniasis in the Americas: etiology, distribution, and clinical and zoonotic importance.Parasit Vectors. 2024 Apr 30;17(1):198. doi: 10.1186/s13071-024-06282-w. Parasit Vectors. 2024. PMID: 38689318 Free PMC article. Review.
-
Two new phlebotomine sandflies (Diptera: Psychodidae) from the forest edge in Madagascar: the anthropophilic Phlebotomus artemievi sp. nov. and Sergentomyia maroantsetra ensis sp. nov.Parasitol Res. 2020 Apr;119(4):1177-1199. doi: 10.1007/s00436-020-06639-x. Epub 2020 Apr 3. Parasitol Res. 2020. PMID: 32246259
-
Infectiousness of Asymptomatic Meriones shawi, Reservoir Host of Leishmania major.Pathogens. 2023 Apr 18;12(4):614. doi: 10.3390/pathogens12040614. Pathogens. 2023. PMID: 37111500 Free PMC article.
-
Non-human primates as indicators of Kinetoplastida diversity in an urban environment in Midwest Brazil.Front Parasitol. 2025 Feb 17;4:1547701. doi: 10.3389/fpara.2025.1547701. eCollection 2025. Front Parasitol. 2025. PMID: 40034868 Free PMC article.
-
Parasites of Free-Ranging and Captive American Primates: A Systematic Review.Microorganisms. 2021 Dec 9;9(12):2546. doi: 10.3390/microorganisms9122546. Microorganisms. 2021. PMID: 34946149 Free PMC article. Review.
References
-
- WHO Expert Committee on the control of leishmaniasis. 2010. http://apps.who.int/iris/bitstream/10665/44412/1/WHO_TRS_949_eng.pdf.
-
- Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil—A Review. Mem Inst Oswaldo Cruz. 2005; 100 (8): 811–827. - PubMed
-
- Patz JA, Githeko AK, McCarty JP, Hussein S, Confaalonieri U, Wet N. Climate change and infectious disease In: McMichel AJ, Campbell-Lendrum DH, Corvalán CF, Ebi KL, Githeko AK, Scheraga JD, Woodwrd A, editors. Climate change and human health Risks and Responses. Genebra: WHO Library; 2003. pp 103–132.
-
- Diniz SA, Silva FL, Carvalho NAV, Bueno R, Guerra RMSNC, Abreu-Silva AL, et al. Animal reservoirs for visceral leishmaniasis in densely populated urban areas. J Infect Dev Ctries. 2008; 2: 24–33. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

