TLR2/4 signaling pathway mediates sperm-induced inflammation in bovine endometrial epithelial cells in vitro
- PMID: 30995239
- PMCID: PMC6469758
- DOI: 10.1371/journal.pone.0214516
TLR2/4 signaling pathway mediates sperm-induced inflammation in bovine endometrial epithelial cells in vitro
Abstract
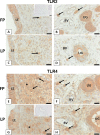
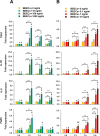
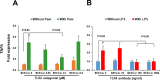
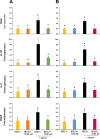
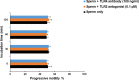
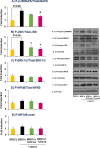
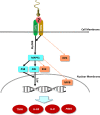
We have recently shown that sperm attachment to bovine endometrial epithelial cells (BEECs) triggers uterine local innate immunity with induction of a pro-inflammatory response in vitro, however details of the mechanism remain unknown. Here, we investigated the involvement of Toll-like receptor 2/4 (TLR2/4) pathway in mediating sperm-BEECs inflammatory process. Immunohistochemistry of the uterine tissue revealed that TLR2 and TLR4 proteins were present in the luminal and glandular epithelia of bovine endometrium. Moreover, BEECs monolayers were treated with TLR2 agonist (Pam; 0, 10, 100, and 1000 ng/ml) or TLR4 agonist (LPS; 0, 0.1, 1, and 10 ng/ml) for 0, 1, 3, or 6 h, followed by evaluating mRNA expression of the pro-inflammatory genes (TNFA, IL-1B, IL-8, and PGES) in BEECs using a real-time PCR. Both Pam and LPS treatments showed a dose-dependent stimulation of mRNA expression of the pro-inflammatory genes. To elucidate the functional role of TLR2/4 in sperm-BEECs interaction, BEECs monolayers were incubated with either TLR2 antagonist or TLR4 antibody for 2 h prior to the co-culture with sperm for 3 h. Importantly, pre-incubation of BEECs with TLR2 antagonist or TLR4 antibody prevented the stimulatory effect of sperm on the transcription of pro-inflammatory genes in BEECs. Furthermore, sperm increased the phosphorylation levels of TLR2/4 downstream targets (p38MAPK and JNK) in BEECs within 1 h of the co-culture. Treatment of BEECs with TLR2 antagonist prior to sperm addition inhibited JNK phosphorylation, while TLR4 antibody inhibited the phosphorylation of both p38MAPK and JNK. In conclusion, the present in vitro findings strongly suggest that bovine endometrial epithelial cells respond to sperm via TLR2/4 signal transduction.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be preserved as prejudicing the impartiality of the research reported.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

