PAR2-Mediated cAMP Generation Suppresses TRPV4-Dependent Ca2+ Signaling in Alveolar Macrophages to Resolve TLR4-Induced Inflammation
- PMID: 30995477
- PMCID: PMC6485424
- DOI: 10.1016/j.celrep.2019.03.053
PAR2-Mediated cAMP Generation Suppresses TRPV4-Dependent Ca2+ Signaling in Alveolar Macrophages to Resolve TLR4-Induced Inflammation
Abstract
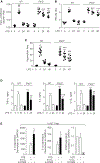
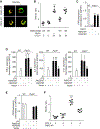
Alveolar macrophages (AMs), upon sensing pathogens, trigger host defense by activating toll-like receptor 4 (TLR4), but the counterbalancing mechanisms that deactivate AM inflammatory signaling and prevent lethal edema, the hallmark of acute lung injury (ALI), remain unknown. Here, we demonstrate the essential role of AM protease-activating receptor 2 (PAR2) in rapidly suppressing inflammation to prevent long-lasting injury. We show that thrombin, released during TLR4-induced lung injury, directly activates PAR2 to generate cAMP, which abolishes Ca2+ entry through the TRPV4 channel. Deletion of PAR2 and thus the accompanying cAMP generation augments Ca2+ entry via TRPV4, causing sustained activation of the transcription factor NFAT to produce long-lasting TLR4-mediated inflammatory lung injury. Rescuing thrombin-sensitive PAR2 expression or blocking TRPV4 activity in PAR2-null AMs restores their capacity to resolve inflammation and reverse lung injury. Thus, activation of the thrombin-induced PAR2-cAMP cascade in AMs suppresses TLR4 inflammatory signaling to reinstate tissue integrity.
Keywords: Ca(2+) entry; NFAT; TRPV4; acute lung injury; alveolar macrophages; cAMP; protease-activating receptor 2.
Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures




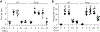
References
-
- Arniges M, Fernández-Fernández JM, Albrecht N, Schaefer M, and Valverde MA (2006). Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J. Biol. Chem 281, 1580–1586. - PubMed
-
- Bair AM, Thippegowda PB, Freichel M, Cheng N, Ye RD, Vogel SM, Yu Y, Flockerzi V, Malik AB, and Tiruppathi C (2009). Ca2+ entry via TRPC channels is necessary for thrombin-induced NF-kappaB activation in endothelial cells through AMP-activated protein kinase and protein kinase Cdelta. J. Biol. Chem 284, 563–574. - PMC - PubMed
-
- Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, et al. (2014). TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol 307, L158–L172. - PMC - PubMed
-
- Ballinger MN, and Christman JW (2016). Pulmonary Macrophages: Overlooked and Underappreciated. Am. J. Respir. Cell Mol. Biol 54, 1–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

