Transforming Growth Factor-β Signaling in Immunity and Cancer
- PMID: 30995507
- PMCID: PMC7507121
- DOI: 10.1016/j.immuni.2019.03.024
Transforming Growth Factor-β Signaling in Immunity and Cancer
Abstract
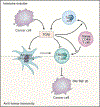
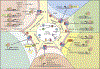
Transforming growth factor (TGF)-β is a crucial enforcer of immune homeostasis and tolerance, inhibiting the expansion and function of many components of the immune system. Perturbations in TGF-β signaling underlie inflammatory diseases and promote tumor emergence. TGF-β is also central to immune suppression within the tumor microenvironment, and recent studies have revealed roles in tumor immune evasion and poor responses to cancer immunotherapy. Here, we present an overview of the complex biology of the TGF-β family and its context-dependent nature. Then, focusing on cancer, we discuss the roles of TGF-β signaling in distinct immune cell types and how this knowledge is being leveraged to unleash the immune system against the tumor.
Copyright © 2019. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests
JM is a scientific advisor and owns company stock in Scholar Rock. EB declares no conflict of interest.
Figures


References
-
- Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, Steele SJ, Roberts RRA, and Heier A (2011). Induction of Heart Valve Lesions by Small-Molecule ALK5 Inhibitors. Toxicol. Pathol. 39, 916–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

