Optimal oxygenation and role of free radicals in PPHN
- PMID: 30995536
- PMCID: PMC6761018
- DOI: 10.1016/j.freeradbiomed.2019.04.001
Optimal oxygenation and role of free radicals in PPHN
Abstract
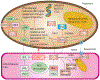
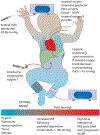
Effective ventilation of the lungs is essential in mediating pulmonary vasodilation at birth to allow effective gas exchange and an increase in systemic oxygenation. Unsuccessful transition prevents the increase in pulmonary blood flow after birth resulting in hypoxemia and persistent pulmonary hypertension of the newborn (PPHN). Management of neonates with PPHN includes ventilation of the lungs with supplemental oxygen to correct hypoxemia. Optimal oxygenation should meet oxygen demand to the tissues and avoid hypoxic pulmonary vasoconstriction (HPV) while preventing oxidative stress. The optimal target for oxygenation in PPHN is not known. Animal models have demonstrated that PaO2<45 mmHg exacerbates HPV. However, there are no practical methods of assessing oxygen levels associated with oxidant stress. Oxidant stress can be due to free radical generation from underlying lung disease or from free radicals generated by supplemental oxygen. Free radicals act on the nitric oxide pathway reducing cGMP and promoting pulmonary vasoconstriction. Antioxidant therapy improves systemic oxygenation in an animal model of PPHN but there are no clinical trials to support such therapy. Targeting preductal SpO2 between 90 and 97% and PaO2 at 50-80 mmHg appears prudent in PPHN but clinical trials to support this practice are lacking. Preterm infants with PPHN present unique challenges due to lack of antioxidant defenses and functional and structural immaturity of the lungs. This review highlights the need for additional studies to mitigate the impact of oxidative stress in the lung and pulmonary vasculature in PPHN.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures

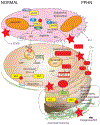

Lambs were ventilated with 100% oxygen for 24 hours irrespective of PaO2 levels.
Lambs were ventilated with 100% oxygen and 20 ppm iNO for 24 hours irrespective of PaO2 levels.
Lambs were ventilated with 100% oxygen and 20 ppm iNO for 24 hours irrespective of PaO2 levels. These lambs received a dose of intratracheal recombinant human superoxide dismutase (rhSOD) mixed with surfactant at birth.
Lambs were ventilated with titrated inspired oxygen to maintain preductal PaO2 between 50 and 80 mmHg and 20 ppm iNO for 24 hours.


Similar articles
-
Pulmonary hypertension and oxidative stress: Where is the link?Semin Fetal Neonatal Med. 2022 Aug;27(4):101347. doi: 10.1016/j.siny.2022.101347. Epub 2022 Apr 19. Semin Fetal Neonatal Med. 2022. PMID: 35473693 Free PMC article. Review.
-
Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn.Am J Physiol Lung Cell Mol Physiol. 2012 Mar 15;302(6):L595-603. doi: 10.1152/ajplung.00145.2011. Epub 2011 Dec 23. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22198909 Free PMC article.
-
Considerations in the management of hypoxemic respiratory failure and persistent pulmonary hypertension in term and late preterm neonates.J Perinatol. 2016 Jun;36 Suppl 2:S12-9. doi: 10.1038/jp.2016.44. J Perinatol. 2016. PMID: 27225960 Review.
-
Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension.Am J Respir Crit Care Med. 2006 Dec 15;174(12):1370-7. doi: 10.1164/rccm.200605-676OC. Epub 2006 Sep 28. Am J Respir Crit Care Med. 2006. PMID: 17008638 Free PMC article.
-
Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn.Respir Physiol Neurobiol. 2010 Dec 31;174(3):272-81. doi: 10.1016/j.resp.2010.08.018. Epub 2010 Sep 6. Respir Physiol Neurobiol. 2010. PMID: 20804862 Free PMC article.
Cited by
-
Effects of nasal high flow on sympathovagal balance, sleep, and sleep-related breathing in patients with precapillary pulmonary hypertension.Sleep Breath. 2021 Jun;25(2):705-717. doi: 10.1007/s11325-020-02159-1. Epub 2020 Aug 22. Sleep Breath. 2021. PMID: 32827122 Free PMC article.
-
Placental Transfusion for Asphyxiated Infants.Front Pediatr. 2019 Nov 20;7:473. doi: 10.3389/fped.2019.00473. eCollection 2019. Front Pediatr. 2019. PMID: 31824895 Free PMC article. Review.
-
Oxidative Stress and Its Implications in the Right Ventricular Remodeling Secondary to Pulmonary Hypertension.Front Physiol. 2019 Sep 24;10:1233. doi: 10.3389/fphys.2019.01233. eCollection 2019. Front Physiol. 2019. PMID: 31607955 Free PMC article. Review.
-
Physiological-based cord clamping versus immediate cord clamping for infants born with a congenital diaphragmatic hernia (PinC): study protocol for a multicentre, randomised controlled trial.BMJ Open. 2022 Mar 18;12(3):e054808. doi: 10.1136/bmjopen-2021-054808. BMJ Open. 2022. PMID: 35304395 Free PMC article.
-
Respiratory Support of Infants With Congenital Diaphragmatic Hernia.Front Pediatr. 2021 Dec 24;9:808317. doi: 10.3389/fped.2021.808317. eCollection 2021. Front Pediatr. 2021. PMID: 35004552 Free PMC article. Review.
References
-
- Lapointe A, Barrington KJ, Pulmonary hypertension and the asphyxiated newborn, The Journal of pediatrics 158(2 Suppl) (2011) e19–24. - PubMed
-
- Lakshminrusimha S, Shankaran S, Laptook A, McDonald S, Keszler M, Van Meurs K, Guillet R, Chawla S, Sood BG, Bonifacio S, Das A, Higgins RD, Pulmonary Hypertension Associated with Hypoxic-Ischemic Encephalopathy-Antecedent Characteristics and Comorbidities, The Journal of pediatrics 196 (2018) 45–51 e3. - PMC - PubMed
-
- Murphy JD, Vawter GF, Reid LM, Pulmonary vascular disease in fatal meconium aspiration, The Journal of pediatrics 104(5) (1984) 758–62. - PubMed
-
- Murphy JD, Rabinovitch M, Goldstein JD, Reid LM, The structural basis of persistent pulmonary hypertension of the newborn infant, The Journal of pediatrics 98(6) (1981) 962–7. - PubMed

