Blood Chromogranin A Is Not Effective as a Biomarker for Diagnosis or Management of Bronchopulmonary Neuroendocrine Tumors/Neoplasms
- PMID: 30995665
- PMCID: PMC7472424
- DOI: 10.1159/000500202
Blood Chromogranin A Is Not Effective as a Biomarker for Diagnosis or Management of Bronchopulmonary Neuroendocrine Tumors/Neoplasms
Abstract
Background: Identification of circulating tumor markers for clinical management in bronchopulmonary (BP) neuroendocrine tumors/neoplasms (NET/NEN) is of considerable clinical interest. Chromogranin A (CgA), a "universal" NET biomarker, is considered controversial as a circulating biomarker of BPNEN.
Aim: Assess utility of CgA in the diagnosis and management of BPNEN in a multicentric study.
Material and methods: CgA diagnostic metrics were assessed in lung NET/NENs (n = 200) and controls (n = 140), randomly assigned to a Training and Test set (100 BPC and 70 controls in each). Assay specificity was evaluated in neoplastic lung disease (n = 137) and nonneoplastic lung disease (n = 77). CgA efficacy in predicting clinical status was evaluated in the combined set of 200 NET/NENs. CgA levels in bronchopulmonary neuroendocrine tumor (BPNET) subtypes (atypical [AC] vs. typical [TC]) and grade was examined. The clinical utility of an alteration of CgA levels (±25%) was evaluated in a subset of 49 BPNET over 12 months. CgA measurement was by NEOLISATM kit (EuroDiagnostica).
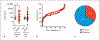
Results: Sensitivity and specificity in the training set were 41/98%, respectively. Test set data were 42/87%. Training set area under receiver operator characteristic analysis differentiated BPC from control area under the curve (AUC) 0.61 ± 0.05 p = 0.015. Test set the data were AUC 0.58 ± 0.05, p = 0.076. In the combined set (n = 200), 67% BPNET/NEN (n = 134) had normal CgA levels. CgA levels did not distinguish histological subtypes (TC vs. AC, AUC 0.56 ± 0.04, p = 0.21), grade (p = 0.45-0.72), or progressive from stable disease (AUC 0.53 ± 0.05 p = 0.47). There was no correlation of CgA with Ki-67 index (Pearson r = 0.143, p = 0.14). For nonneoplastic diseases (chronic obstructive pulmonary disorder and idiopathic pulmonary fibrosis), CgA was elevated in 26-37%. For neoplastic disease (NSCLC, squamous cell carcinoma), CgA was elevated in 11-16%. The neuroendocrine SCLC also exhibited elevated CgA (50%). Elevated CgA was not useful for differentiating BPNET/NEN from these other pathologies. Monitoring BPNET/NEN over a 12-month period identified neither CgA levels per se nor changes in CgA were reflective of somatostatin analog treatment outcome/efficacy or the natural history of the disease (progression).
Conclusions: Blood CgA levels are not clinically useful as a biomarker for lung BPNET/NEN. The low specificity and elevations in both nonneoplastic as well as other common neoplastic lung diseases identified limited clinical utility for this biomarker.
Keywords: Biomarker; Bronchopulmonary; Carcinoid; Chromogranin A; Diagnosis; Lung; Prognosis.
© 2019 S. Karger AG, Basel.
Figures
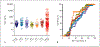

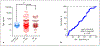

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

