Sensors that Learn: The Evolution from Taste Fingerprints to Patterns of Early Disease Detection
- PMID: 30995728
- PMCID: PMC6523560
- DOI: 10.3390/mi10040251
Sensors that Learn: The Evolution from Taste Fingerprints to Patterns of Early Disease Detection
Abstract
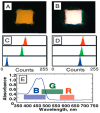
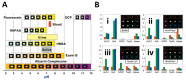
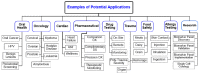
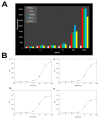
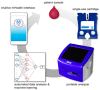
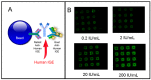
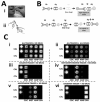
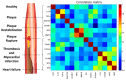
The McDevitt group has sustained efforts to develop a programmable sensing platform that offers advanced, multiplexed/multiclass chem-/bio-detection capabilities. This scalable chip-based platform has been optimized to service real-world biological specimens and validated for analytical performance. Fashioned as a sensor that learns, the platform can host new content for the application at hand. Identification of biomarker-based fingerprints from complex mixtures has a direct linkage to e-nose and e-tongue research. Recently, we have moved to the point of big data acquisition alongside the linkage to machine learning and artificial intelligence. Here, exciting opportunities are afforded by multiparameter sensing that mimics the sense of taste, overcoming the limitations of salty, sweet, sour, bitter, and glutamate sensing and moving into fingerprints of health and wellness. This article summarizes developments related to the electronic taste chip system evolving into a platform that digitizes biology and affords clinical decision support tools. A dynamic body of literature and key review articles that have contributed to the shaping of these activities are also highlighted. This fully integrated sensor promises more rapid transition of biomarker panels into wide-spread clinical practice yielding valuable new insights into health diagnostics, benefiting early disease detection.
Keywords: biomarkers; biosensors; early disease detection; electronic taste chip; electronic tongue; point-of-care; programmable bio-nano-chip (p-BNC); saliva; serum.
Conflict of interest statement
Principal Investigator, John T. McDevitt, has an equity interest in SensoDx II, LLC. He also serves on the Scientific Advisory Board of SensoDx. Michael P. McRae has served as a consultant for SensoDx.
Figures


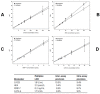
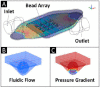

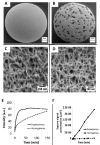
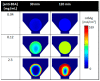





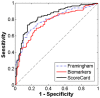

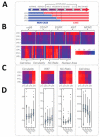



References
-
- Vitzthum F., Behrens F., Anderson N.L., Shaw J.H. Proteomics: From basic research to diagnostic application. A review of requirements & needs. J. Prot. Res. 2005;4:1086–1097. - PubMed
-
- Chin C., Chin S., Laksanasopin T., Sia S. Low-cost microdevices for point-of-care testing. In: Issadore D., Westervelt R.M., editors. Point-of-Care Diagnostics on a Chip. Springer; Berlin/Heidelberg, Germany: 2013. pp. 3–21.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

