Anticancer Effects of Five Biflavonoids from Ginkgo Biloba L. Male Flowers In Vitro
- PMID: 30995808
- PMCID: PMC6514578
- DOI: 10.3390/molecules24081496
Anticancer Effects of Five Biflavonoids from Ginkgo Biloba L. Male Flowers In Vitro
Abstract
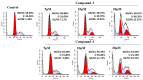
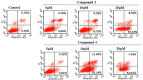
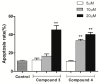
Ginkgo biloba L., an ancient dioecious gymnosperm, is now cultivated worldwide for landscaping and medical purposes. A novel biflavonoid-amentoflavone 7''-O-β-D-glucopyranoside (1)-and four known biflavonoids were isolated and identified from the male flowers of Ginkgo. The anti-proliferative activities of five biflavonoids were evaluated on different cancer lines. Bilobetin (3) and isoginkgetin (4) exhibited better anti-proliferative activities on different cancer lines. Their effects were found to be cell-specific and in a dose and time dependent manner for the most sensitive HeLa cells. The significant morphological changes validated their anticancer effects in a dose-dependent manner. They were capable of arresting the G2/M phase of the cell cycle, inducing the apoptosis of HeLa cells dose-dependently and activating the proapoptotic protein Bax and the executor caspase-3. Bilobetin (3) could also inhibit the antiapoptotic protein Bcl-2. These might be the mechanism underlying their anti-proliferation. In short, bilobetin (3) and isoginkgetin (4) might be the early lead compounds for new anticancer agents.
Keywords: Ginkgo biloba flowers; anticancer; biflavonoids; bilobetin; isoginkgetin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
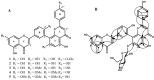
 ) and nuclear overhauser enhancement spectroscopy (NOESY)correlations (
) and nuclear overhauser enhancement spectroscopy (NOESY)correlations ( ) of compound 1 (B).
) of compound 1 (B).


References
-
- Du H., Huang Y., Hou X., Quan X., Jiang J., Wei X., Liu Y., Li H., Wang P., Zhan M., et al. Two novel camptothecin derivatives inhibit colorectal cancer proliferation via induction of cell cycle arrest and apoptosis in vitro and in vivo. Eur J Pharm Sci. 2018;123:546–559. doi: 10.1016/j.ejps.2018.08.018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

