Therapeutic novelties in migraine: new drugs, new hope?
- PMID: 30995909
- PMCID: PMC6734360
- DOI: 10.1186/s10194-019-0974-3
Therapeutic novelties in migraine: new drugs, new hope?
Erratum in
-
Correction to: Therapeutic novelties in migraine: new drugs, new hope?J Headache Pain. 2019 May 17;20(1):55. doi: 10.1186/s10194-019-1013-0. J Headache Pain. 2019. PMID: 31100999 Free PMC article.
Abstract
Background: In the past decade, migraine research has identified novel drug targets. In this review, we discuss recent data on emerging anti-migraine therapies.
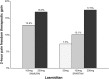
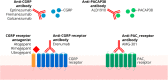
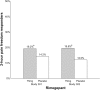
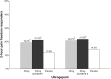
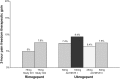
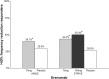
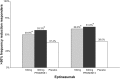
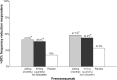
Main body: The development of ditans, gepants and anti-calcitonin gene-related peptide monoclonal antibodies for the treatment of migraine is one of the greatest advances in the migraine field. Lasmiditan, rimegepant and ubrogepant will extend our therapeutic armamentarium for managing acute migraine attacks when triptans are not effective or contraindicated due to cardiovascular disorders. The monoclonal antibodies are migraine specific prophylactic drugs with high responder rates and favorable adverse event profiles. Furthermore, they offer convenient treatment regimens of 4- or 12-week intervals.
Conclusion: Collectively, novel migraine therapies represent a major progress in migraine treatment and will undoubtedly transform headache medicine.
Keywords: Adverse event; Antibody; Ditan; Efficacy; Gepant; Migraine; Randomized clinical trial; Tolerability.
Conflict of interest statement
MA is a consultant or scientific advisor for Allergan, Amgen, Alder, Eli Lilly, Novartis and Teva, principal investigator for: Amgen 20,120,178 (Phase 2), 20,120,295 (Phase 2), 20,130,255 (Open label extension), 20,120,297 (Phase 3), 20,150,308 (Phase 2), ElectroCore GM-11 gamma-Core-R, TEVA TV48125-CNS-30068 (Phase 3), Novartis CAMG334A2301 (Phase 3) and Alder PROMISE-2. MA has no ownership interest and does not hold stock in any pharmaceutical company. MA serves as associated editor of Cephalalgia and co-editor of the Journal of Headache and Pain and is Editor for the thematic series 'The changing face of migraine'. TPD and SG report no competing interests.
Figures

Similar articles
-
The pharmacotherapeutic management of episodic and chronic migraine with gepants.Expert Opin Pharmacother. 2023 Jun;24(8):947-958. doi: 10.1080/14656566.2023.2201375. Epub 2023 Apr 12. Expert Opin Pharmacother. 2023. PMID: 37038933 Review.
-
[New therapeutic era for migraine attacks with recently approved monoclonal antibodies, ditans and gepants].Rev Neurol. 2024 Jan 16;78(2):47-57. doi: 10.33588/rn.7802.2023176. Rev Neurol. 2024. PMID: 38223948 Free PMC article. Review. Spanish.
-
Gepants, calcitonin-gene-related peptide receptor antagonists: what could be their role in migraine treatment?Curr Opin Neurol. 2020 Jun;33(3):309-315. doi: 10.1097/WCO.0000000000000806. Curr Opin Neurol. 2020. PMID: 32251023 Review.
-
Novel synthetic treatment options for migraine.Expert Opin Pharmacother. 2021 May;22(7):907-922. doi: 10.1080/14656566.2020.1862793. Epub 2020 Dec 28. Expert Opin Pharmacother. 2021. PMID: 33369482 Review.
-
Diagnosis and Management of Headache: A Review.JAMA. 2021 May 11;325(18):1874-1885. doi: 10.1001/jama.2021.1640. JAMA. 2021. PMID: 33974014 Review.
Cited by
-
A Comprehensive Review of the Mechanism, Efficacy, Safety, and Tolerability of Ubrogepant in the Treatment of Migraine.Cureus. 2023 Nov 2;15(11):e48160. doi: 10.7759/cureus.48160. eCollection 2023 Nov. Cureus. 2023. PMID: 38046695 Free PMC article. Review.
-
Revisiting Migraine: The Evolving Pathophysiology and the Expanding Management Armamentarium.Cureus. 2023 Feb 2;15(2):e34553. doi: 10.7759/cureus.34553. eCollection 2023 Feb. Cureus. 2023. PMID: 36879707 Free PMC article. Review.
-
Monoclonal anti-CGRP antibodies in post-menopausal women: a real-life study.Acta Neurol Belg. 2023 Jun;123(3):1039-1047. doi: 10.1007/s13760-023-02190-5. Epub 2023 Mar 3. Acta Neurol Belg. 2023. PMID: 36867346
-
Proteome-wide Mendelian randomization identified potential drug targets for migraine.J Headache Pain. 2024 Sep 11;25(1):148. doi: 10.1186/s10194-024-01853-9. J Headache Pain. 2024. PMID: 39261750 Free PMC article.
-
Upholding or Breaking the Law of Superposition in Pharmacokinetics.Biomedicines. 2024 Aug 13;12(8):1843. doi: 10.3390/biomedicines12081843. Biomedicines. 2024. PMID: 39200307 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

