Nox2 NADPH oxidase is dispensable for platelet activation or arterial thrombosis in mice
- PMID: 30995985
- PMCID: PMC6482355
- DOI: 10.1182/bloodadvances.2018025569
Nox2 NADPH oxidase is dispensable for platelet activation or arterial thrombosis in mice
Abstract
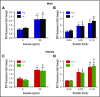
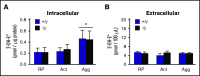
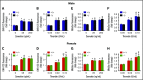
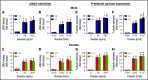
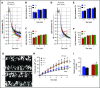
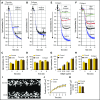
Deficiency of the Nox2 (gp91phox) catalytic subunit of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a genetic cause of X-linked chronic granulomatous disease, a condition in which patients are prone to infection resulting from the loss of oxidant production by neutrophils. Some studies have suggested a role for superoxide derived from Nox2 NADPH oxidase in platelet activation and thrombosis, but data are conflicting. Using a rigorous and comprehensive approach, we tested the hypothesis that genetic deficiency of Nox2 attenuates platelet activation and arterial thrombosis. Our study was designed to test the genotype differences within male and female mice. Using chloromethyl-dichlorodihydrofluorescein diacetate, a fluorescent dye, as well as high-performance liquid chromatography analysis with dihydroethidium as a probe to detect intracellular reactive oxygen species (ROS), we observed no genotype differences in ROS levels in platelets. Similarly, there were no genotype-dependent differences in levels of mitochondrial ROS. In addition, we did not observe any genotype-associated differences in platelet activation, adhesion, secretion, or aggregation in male or female mice. Platelets from chronic granulomatous disease patients exhibited similar adhesion and aggregation responses as platelets from healthy subjects. Susceptibility to carotid artery thrombosis in a photochemical injury model was similar in wild-type and Nox2-deficient male or female mice. Our findings indicate that Nox2 NADPH oxidase is not an essential source of platelet ROS or a mediator of platelet activation or arterial thrombosis in large vessels, such as the carotid artery.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Dinauer MC, Orkin SH, Brown R, Jesaitis AJ, Parkos CA. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature. 1987;327(6124):717-720. - PubMed
-
- Liu Y, Hu M, Luo D, et al. . Class III PI3K Positively Regulates Platelet Activation and Thrombosis via PI(3)P-Directed Function of NADPH Oxidase. Arterioscler Thromb Vasc Biol. 2017;37(11):2075-2086. - PubMed
-
- Seno T, Inoue N, Gao D, et al. . Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb Res. 2001;103(5):399-409. - PubMed
-
- Dharmarajah J, Arthur JF, Sobey CG, Drummond GR. The anti-platelet effects of apocynin in mice are not mediated by inhibition of NADPH oxidase activity. Naunyn Schmiedebergs Arch Pharmacol. 2010;382(4):377-384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

