In utero gene editing for monogenic lung disease
- PMID: 30996081
- PMCID: PMC6822403
- DOI: 10.1126/scitranslmed.aav8375
In utero gene editing for monogenic lung disease
Abstract
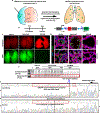
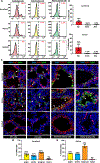
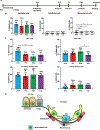
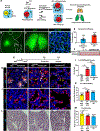
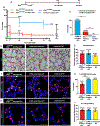
Monogenic lung diseases that are caused by mutations in surfactant genes of the pulmonary epithelium are marked by perinatal lethal respiratory failure or chronic diffuse parenchymal lung disease with few therapeutic options. Using a CRISPR fluorescent reporter system, we demonstrate that precisely timed in utero intra-amniotic delivery of CRISPR-Cas9 gene editing reagents during fetal development results in targeted and specific gene editing in fetal lungs. Pulmonary epithelial cells are predominantly targeted in this approach, with alveolar type 1, alveolar type 2, and airway secretory cells exhibiting high and persistent gene editing. We then used this in utero technique to evaluate a therapeutic approach to reduce the severity of the lethal interstitial lung disease observed in a mouse model of the human SFTPCI73T mutation. Embryonic expression of SftpcI73T alleles is characterized by severe diffuse parenchymal lung damage and rapid demise of mutant mice at birth. After in utero CRISPR-Cas9-mediated inactivation of the mutant SftpcI73T gene, fetuses and postnatal mice showed improved lung morphology and increased survival. These proof-of-concept studies demonstrate that in utero gene editing is a promising approach for treatment and rescue of monogenic lung diseases that are lethal at birth.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
Comment in
-
Recent Insights into In Utero Gene Editing, Checkpoint Inhibitors, and Polymorphonuclear Extracellular Vesicles.Am J Respir Cell Mol Biol. 2020 Jun;62(6):805-807. doi: 10.1165/rcmb.2020-0024RO. Am J Respir Cell Mol Biol. 2020. PMID: 32017596 No abstract available.
References
-
- Hamvas A, Cole FS, Nogee LM, Genetic disorders of surfactant proteins. Neonatology 91, 311–317 (2007). - PubMed
-
- Rowe SM, Miller S, Sorscher EJ, Cystic fibrosis. N. Engl. J. Med 352, 1992–2001 (2005). - PubMed
-
- Spoonhower KA, Davis PB, Epidemiology of cystic fibrosis. Clin. Chest Med 37, 1–8 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

