Enterovirus A71 Containing Codon-Deoptimized VP1 and High-Fidelity Polymerase as Next-Generation Vaccine Candidate
- PMID: 30996087
- PMCID: PMC6580961
- DOI: 10.1128/JVI.02308-18
Enterovirus A71 Containing Codon-Deoptimized VP1 and High-Fidelity Polymerase as Next-Generation Vaccine Candidate
Abstract
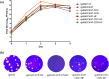
Enterovirus A71 (EV-A71) is a major pathogen that causes hand-foot-and-mouth disease (HFMD), which occasionally results in severe neurological complications. In this study, we developed four EV-A71 (rgEV-A71) strains by reverse genetics procedures as possible vaccine candidates. The four rgEV-A71 viruses contained various codon-deoptimized VP1 capsid proteins (VP1-CD) and showed replication rates and antigenicity similar to that of the wild-type virus, while a fifth virus, rg4643C4VP-CD, was unable to form plaques but was still able to be examined by median tissue culture infectious dose (TCID50) titers, which were similar to those of the others, indicating the effect of CD on plaque formation. However, the genome stability showed that there were some mutations which appeared during just one passage of the VP1-CD viruses. Thus, we further constructed VP1-CD rgEV-A71 containing high-fidelity determinants in 3D polymerase (CD-HF), and the number of mutations in CD-HF rgEV-A71 was shown to have decreased. The CD-HF viruses showed less virulence than the parental strain in a mouse infection model. After 14 days postimmunization, antibody titers had increased in mice infected with CD-HF viruses. The mouse antisera showed similar neutralizing antibody titers against various CD-HF viruses and different genotypes of EV-A71. The study demonstrates the proof of concept that VP1 codon deoptimization combined with high-fidelity 3D polymerase decreased EV-A71 mutations and virulence in mice but retained their antigenicity, indicating it is a good candidate for next-generation EV-A71 vaccine development.IMPORTANCE EV-A71 can cause severe neurological diseases with fatality in infants and young children, but there are still no effective drugs to date. Here, we developed a novel vaccine strategy with the combination of CD and HF substitutions to generate the genetically stable reverse genetics virus. We found that CD combined with HF polymerase decreased the virulence but maintained the antigenicity of the virus. This work demonstrated the simultaneous introduction of CD genome sequences and HF substitutions as a potential new strategy to develop attenuated vaccine seed virus. Our work provides insight into the development of a low-virulence candidate vaccine virus through a series of genetic editing of virus sequences while maintaining its antigenicity and genome stability, which will provide an additional strategy for next-generation vaccine development of EV-A71.
Keywords: codon deoptimization; enterovirus A71; high fidelity; reverse genetics virus; vaccine.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
VP1 codon deoptimization and high-fidelity substitutions in 3D polymerase as potential vaccine strategies for eliciting immune responses against enterovirus A71.J Virol. 2024 Jan 23;98(1):e0155823. doi: 10.1128/jvi.01558-23. Epub 2024 Jan 4. J Virol. 2024. PMID: 38174926 Free PMC article.
-
Vaccine candidates generated by codon and codon pair deoptimization of enterovirus A71 protect against lethal challenge in mice.Vaccine. 2021 Mar 19;39(12):1708-1720. doi: 10.1016/j.vaccine.2021.02.024. Epub 2021 Feb 25. Vaccine. 2021. PMID: 33640144
-
Development of a full-length cDNA-derived enterovirus A71 vaccine candidate using reverse genetics technology.Antiviral Res. 2016 Aug;132:225-32. doi: 10.1016/j.antiviral.2016.06.014. Epub 2016 Jul 4. Antiviral Res. 2016. PMID: 27387826
-
Impact of genetic changes, pathogenicity and antigenicity on Enterovirus- A71 vaccine development.Virology. 2017 Jun;506:121-129. doi: 10.1016/j.virol.2017.03.017. Epub 2017 Apr 4. Virology. 2017. PMID: 28384566 Review.
-
EV-A71 vaccine licensure: a first step for multivalent enterovirus vaccine to control HFMD and other severe diseases.Emerg Microbes Infect. 2016 Jul 20;5(7):e75. doi: 10.1038/emi.2016.73. Emerg Microbes Infect. 2016. PMID: 27436364 Free PMC article. Review.
Cited by
-
Sequence analysis of SARS-CoV-2 genome reveals features important for vaccine design.Sci Rep. 2020 Sep 24;10(1):15643. doi: 10.1038/s41598-020-72533-2. Sci Rep. 2020. PMID: 32973171 Free PMC article.
-
Recurrent Hand, Foot, and Mouth Disease in a Saudi Girl.Cureus. 2024 Jan 7;16(1):e51813. doi: 10.7759/cureus.51813. eCollection 2024 Jan. Cureus. 2024. PMID: 38322079 Free PMC article.
-
Viruses as tools in gene therapy, vaccine development, and cancer treatment.Arch Virol. 2022 Jun;167(6):1387-1404. doi: 10.1007/s00705-022-05432-8. Epub 2022 Apr 24. Arch Virol. 2022. PMID: 35462594 Free PMC article. Review.
-
Development of an Enterovirus 71 Vaccine Efficacy Test Using Human Scavenger Receptor B2 Transgenic Mice.J Virol. 2020 Feb 28;94(6):e01921-19. doi: 10.1128/JVI.01921-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31896594 Free PMC article.
-
Altering Compositional Properties of Viral Genomes to Design Live-Attenuated Vaccines.Front Microbiol. 2021 Jun 30;12:676582. doi: 10.3389/fmicb.2021.676582. eCollection 2021. Front Microbiol. 2021. PMID: 34276608 Free PMC article. Review.
References
-
- Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht H-J, Johnson JE, Kamer G, Luo M, Mosser AG, Rueckert RR, Sherry B, Vriend G. 1985. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature 317:145–153. doi:10.1038/317145a0. - DOI - PubMed
-
- Ma HC, Liu Y, Wang C, Strauss M, Rehage N, Chen YH, Altan-Bonnet N, Hogle J, Wimmer E, Mueller S, Paul AV, Jiang P. 2014. An interaction between glutathione and the capsid is required for the morphogenesis of C-cluster enteroviruses. PLoS Pathog 10:e1004052. doi:10.1371/journal.ppat.1004052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

