Cancer stemness, intratumoral heterogeneity, and immune response across cancers
- PMID: 30996127
- PMCID: PMC6500180
- DOI: 10.1073/pnas.1818210116
Cancer stemness, intratumoral heterogeneity, and immune response across cancers
Abstract
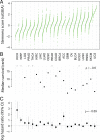
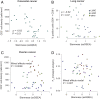
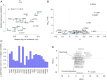
Regulatory programs that control the function of stem cells are active in cancer and confer properties that promote progression and therapy resistance. However, the impact of a stem cell-like tumor phenotype ("stemness") on the immunological properties of cancer has not been systematically explored. Using gene-expression-based metrics, we evaluated the association of stemness with immune cell infiltration and genomic, transcriptomic, and clinical parameters across 21 solid cancers. We found pervasive negative associations between cancer stemness and anticancer immunity. This occurred despite high stemness cancers exhibiting increased mutation load, cancer-testis antigen expression, and intratumoral heterogeneity. Stemness was also strongly associated with cell-intrinsic suppression of endogenous retroviruses and type I IFN signaling, and increased expression of multiple therapeutically accessible immunosuppressive pathways. Thus, stemness is not only a fundamental process in cancer progression but may provide a mechanistic link between antigenicity, intratumoral heterogeneity, and immune suppression across cancers.
Keywords: antitumor immunity; cancer stemness; intratumoral heterogeneity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
SERPINA12 promotes the tumorigenic capacity of HCC stem cells through hyperactivation of AKT/β-catenin signaling.Hepatology. 2023 Dec 1;78(6):1711-1726. doi: 10.1097/HEP.0000000000000269. Epub 2023 Jan 13. Hepatology. 2023. PMID: 36630996
References
-
- Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–1134. - PubMed
-
- Nassar D, Blanpain C. Cancer stem cells: Basic concepts and therapeutic implications. Annu Rev Pathol. 2016;11:47–76. - PubMed
-
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

