The female-biased factor VGLL3 drives cutaneous and systemic autoimmunity
- PMID: 30996136
- PMCID: PMC6538382
- DOI: 10.1172/jci.insight.127291
The female-biased factor VGLL3 drives cutaneous and systemic autoimmunity
Abstract
Autoimmune disease is 4 times more common in women than men. This bias is largely unexplained. Female skin is "autoimmunity prone," showing upregulation of many proinflammatory genes, even in healthy women. We previously identified VGLL3 as a putative transcription cofactor enriched in female skin. Here, we demonstrate that skin-directed overexpression of murine VGLL3 causes a severe lupus-like rash and systemic autoimmune disease that involves B cell expansion, autoantibody production, immune complex deposition, and end-organ damage. Excess epidermal VGLL3 drives a proinflammatory gene expression program that overlaps with both female skin and cutaneous lupus. This includes increased B cell-activating factor (BAFF), the only current biologic target in systemic lupus erythematosus (SLE); IFN-κ, a key inflammatory mediator in cutaneous lupus; and CXCL13, a biomarker of early-onset SLE and renal involvement. Our results demonstrate that skin-targeted overexpression of the female-biased factor VGLL3 is sufficient to drive cutaneous and systemic autoimmune disease that is strikingly similar to SLE. This work strongly implicates VGLL3 as a pivotal orchestrator of sex-biased autoimmunity.
Keywords: Autoimmune diseases; Autoimmunity; Dermatology; Lupus; Mouse models.
Conflict of interest statement
Figures

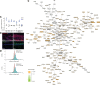


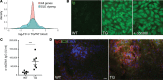
References
-
- Ramírez A, Bravo A, Jorcano JL, Vidal M. Sequences 5’ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58(1):53–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR073196/AR/NIAMS NIH HHS/United States
- R01 AI172944/AI/NIAID NIH HHS/United States
- R01 AI130025/AI/NIAID NIH HHS/United States
- R21 AR063852/AR/NIAMS NIH HHS/United States
- S10 OD020053/OD/NIH HHS/United States
- P50 AR070590/AR/NIAMS NIH HHS/United States
- T32 AR007197/AR/NIAMS NIH HHS/United States
- R01 AR063437/AR/NIAMS NIH HHS/United States
- P30 DK081943/DK/NIDDK NIH HHS/United States
- R01 AR069071/AR/NIAMS NIH HHS/United States
- R01 AR062546/AR/NIAMS NIH HHS/United States
- R01 AR071384/AR/NIAMS NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- P30 AR075043/AR/NIAMS NIH HHS/United States
- K01 AR072129/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

