ITGA2 as a potential nanotherapeutic target for glioblastoma
- PMID: 30996239
- PMCID: PMC6470144
- DOI: 10.1038/s41598-019-42643-7
ITGA2 as a potential nanotherapeutic target for glioblastoma
Abstract
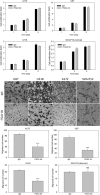
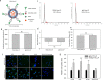
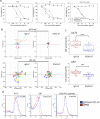
High grade gliomas, including glioblastoma (GBM), are the most common and deadly brain cancers in adults. Here, we performed a quantitative and unbiased screening of 70 cancer-related antigens using comparative flow cytometry and, for the first time, identified integrin alpha-2 (ITGA2) as a novel molecular target for GBM. In comparison to epidermal growth factor receptor (EGFR), a well-established GBM target, ITGA2 is significantly more expressed on human GBM cells and significantly less expressed on normal human glial cells. We also found that ITGA2 antibody blockade significantly impedes GBM cell migration but not GBM cell proliferation. To investigate the utility of ITGA2 as a therapeutic target in GBM, we designed and engineered an ITGA2 antibody-directed liposome that can selectively deliver doxorubicin, a standard-of-care chemotherapeutic agent, to GBM cells. This novel approach significantly improved antitumor efficacy. We also demonstrated that these ITGA2 antibody-directed liposomes can effectively breach the blood-brain tumor barrier (BBTB) in vitro via GBM-induced angiogenesis effects. These findings support further research into the use of ITGA2 as a novel nanotherapeutic target for GBM.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model.Colloids Surf B Biointerfaces. 2019 Jan 1;173:27-35. doi: 10.1016/j.colsurfb.2018.09.047. Epub 2018 Sep 21. Colloids Surf B Biointerfaces. 2019. PMID: 30261346 Free PMC article.
-
Overcoming the blood-brain tumor barrier for effective glioblastoma treatment.Drug Resist Updat. 2015 Mar;19:1-12. doi: 10.1016/j.drup.2015.02.002. Epub 2015 Mar 6. Drug Resist Updat. 2015. PMID: 25791797 Review.
-
Anti-SEMA3A Antibody: A Novel Therapeutic Agent to Suppress Glioblastoma Tumor Growth.Cancer Res Treat. 2018 Jul;50(3):1009-1022. doi: 10.4143/crt.2017.315. Epub 2017 Nov 10. Cancer Res Treat. 2018. PMID: 29129044 Free PMC article.
-
A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment.Drug Deliv. 2019 Dec;26(1):551-565. doi: 10.1080/10717544.2019.1616235. Drug Deliv. 2019. PMID: 31928355 Free PMC article. Review.
-
Muscone restores anoikis sensitivity in TMZ-resistant glioblastoma cells by suppressing TOP2A via the EGFR/Integrin β1/FAK signaling pathway.Phytomedicine. 2024 Jul;129:155714. doi: 10.1016/j.phymed.2024.155714. Epub 2024 May 5. Phytomedicine. 2024. PMID: 38723526
Cited by
-
Integrin α10-Antibodies Reduce Glioblastoma Tumor Growth and Cell Migration.Cancers (Basel). 2021 Mar 9;13(5):1184. doi: 10.3390/cancers13051184. Cancers (Basel). 2021. PMID: 33803359 Free PMC article.
-
Prolactin and its receptor as therapeutic targets in glioblastoma multiforme.Sci Rep. 2019 Dec 20;9(1):19578. doi: 10.1038/s41598-019-55860-x. Sci Rep. 2019. PMID: 31862900 Free PMC article.
-
Recent Advances on Surface-Modified GBM Targeted Nanoparticles: Targeting Strategies and Surface Characterization.Int J Mol Sci. 2023 Jan 27;24(3):2496. doi: 10.3390/ijms24032496. Int J Mol Sci. 2023. PMID: 36768820 Free PMC article. Review.
-
Immune response recalibration using immune therapy and biomimetic nano-therapy against high-grade gliomas and brain metastases.Asian J Pharm Sci. 2025 Apr;20(2):101021. doi: 10.1016/j.ajps.2025.101021. Epub 2025 Jan 17. Asian J Pharm Sci. 2025. PMID: 40224727 Free PMC article. Review.
-
Challenges and Perspectives of Standard Therapy and Drug Development in High-Grade Gliomas.Molecules. 2021 Feb 22;26(4):1169. doi: 10.3390/molecules26041169. Molecules. 2021. PMID: 33671796 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

