Bacterial glycosyltransferase-mediated cell-surface chemoenzymatic glycan modification
- PMID: 30996301
- PMCID: PMC6470217
- DOI: 10.1038/s41467-019-09608-w
Bacterial glycosyltransferase-mediated cell-surface chemoenzymatic glycan modification
Abstract
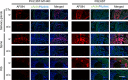
Chemoenzymatic modification of cell-surface glycan structures has emerged as a complementary approach to metabolic oligosaccharide engineering. Here, we identify Pasteurella multocida α2-3-sialyltransferase M144D mutant, Photobacterium damsela α2-6-sialyltransferase, and Helicobacter mustelae α1-2-fucosyltransferase, as efficient tools for live-cell glycan modification. Combining these enzymes with Helicobacter pylori α1-3-fucosyltransferase, we develop a host-cell-based assay to probe glycan-mediated influenza A virus (IAV) infection including wild-type and mutant strains of H1N1 and H3N2 subtypes. At high NeuAcα2-6-Gal levels, the IAV-induced host-cell death is positively correlated with haemagglutinin (HA) binding affinity to NeuAcα2-6-Gal. Remarkably, an increment of host-cell-surface sialyl Lewis X (sLeX) exacerbates the killing by several wild-type IAV strains and a previously engineered mutant HK68-MTA. Structural alignment of HAs from HK68 and HK68-MTA suggests formation of a putative hydrogen bond between Trp222 of HA-HK68-MTA and the C-4 hydroxyl group of the α1-3-linked fucose of sLeX, which may account for the enhanced host cell killing of that mutant.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Mutations flanking the carbohydrate binding site of surfactant protein D confer antiviral activity for pandemic influenza A viruses.Am J Physiol Lung Cell Mol Physiol. 2014 Jun 1;306(11):L1036-44. doi: 10.1152/ajplung.00035.2014. Epub 2014 Apr 4. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24705721 Free PMC article.
-
Human Influenza Virus Hemagglutinins Contain Conserved Oligomannose N-Linked Glycans Allowing Potent Neutralization by Lectins.Cell Host Microbe. 2020 May 13;27(5):725-735.e5. doi: 10.1016/j.chom.2020.03.009. Epub 2020 Apr 15. Cell Host Microbe. 2020. PMID: 32298658 Free PMC article.
-
The monoclonal antibody CHO-131 binds to a core 2 O-glycan terminated with sialyl-Lewis x, which is a functional glycan ligand for P-selectin.Blood. 2002 Jun 1;99(11):4063-9. doi: 10.1182/blood-2001-12-0265. Blood. 2002. PMID: 12010808
-
H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation.Hum Vaccin Immunother. 2018;14(8):1840-1847. doi: 10.1080/21645515.2018.1462639. Epub 2018 May 14. Hum Vaccin Immunother. 2018. PMID: 29641358 Free PMC article. Review.
-
[Swine influenza virus: evolution mechanism and epidemic characterization--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
Cited by
-
Direct Visualization of Live Zebrafish Glycans via Single-Step Metabolic Labeling with Fluorophore-Tagged Nucleotide Sugars.Angew Chem Int Ed Engl. 2019 Oct 1;58(40):14327-14333. doi: 10.1002/anie.201907410. Epub 2019 Aug 28. Angew Chem Int Ed Engl. 2019. PMID: 31295389 Free PMC article. Review.
-
Human T cell glycosylation and implications on immune therapy for cancer.Hum Vaccin Immunother. 2020 Oct 2;16(10):2374-2388. doi: 10.1080/21645515.2020.1730658. Epub 2020 Mar 18. Hum Vaccin Immunother. 2020. PMID: 32186959 Free PMC article. Review.
-
The multifaceted roles of mucins family in lung cancer: from prognostic biomarkers to promising targets.Front Immunol. 2025 Jun 27;16:1608140. doi: 10.3389/fimmu.2025.1608140. eCollection 2025. Front Immunol. 2025. PMID: 40655139 Free PMC article. Review.
-
Cellular and Molecular Engineering of Glycan Sialylation in Heterologous Systems.Molecules. 2021 Sep 30;26(19):5950. doi: 10.3390/molecules26195950. Molecules. 2021. PMID: 34641494 Free PMC article. Review.
-
Aglycone sterics-selective enzymatic glycan remodeling.iScience. 2022 Jun 13;25(7):104578. doi: 10.1016/j.isci.2022.104578. eCollection 2022 Jul 15. iScience. 2022. PMID: 35789841 Free PMC article.
References
-
- Paulson JC, Sadler JE, Hill RL. Restoration of specific myxovirus receptors to asialoerythrocytes by incorporation of sialic acid with pure sialyltransferases. J. Biol. Chem. 1979;254:2120–2124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

