Restoration of brain circulation and cellular functions hours post-mortem
- PMID: 30996318
- PMCID: PMC6844189
- DOI: 10.1038/s41586-019-1099-1
Restoration of brain circulation and cellular functions hours post-mortem
Abstract
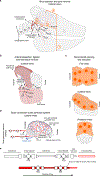
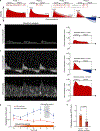
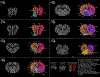
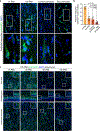
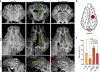
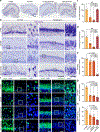
The brains of humans and other mammals are highly vulnerable to interruptions in blood flow and decreases in oxygen levels. Here we describe the restoration and maintenance of microcirculation and molecular and cellular functions of the intact pig brain under ex vivo normothermic conditions up to four hours post-mortem. We have developed an extracorporeal pulsatile-perfusion system and a haemoglobin-based, acellular, non-coagulative, echogenic, and cytoprotective perfusate that promotes recovery from anoxia, reduces reperfusion injury, prevents oedema, and metabolically supports the energy requirements of the brain. With this system, we observed preservation of cytoarchitecture; attenuation of cell death; and restoration of vascular dilatory and glial inflammatory responses, spontaneous synaptic activity, and active cerebral metabolism in the absence of global electrocorticographic activity. These findings demonstrate that under appropriate conditions the isolated, intact large mammalian brain possesses an underappreciated capacity for restoration of microcirculation and molecular and cellular activity after a prolonged post-mortem interval.
Conflict of interest statement
Figures












Comment in
-
Pig experiment challenges assumptions around brain damage in people.Nature. 2019 Apr;568(7752):302-304. doi: 10.1038/d41586-019-01169-8. Nature. 2019. PMID: 30996309 No abstract available.
-
Part-revived pig brains raise slew of ethical quandaries.Nature. 2019 Apr;568(7752):299-302. doi: 10.1038/d41586-019-01168-9. Nature. 2019. PMID: 30996311 No abstract available.
-
Pig brains kept alive outside body for hours after death.Nature. 2019 Apr;568(7752):283-284. doi: 10.1038/d41586-019-01216-4. Nature. 2019. PMID: 30996321 No abstract available.
-
Preserving perfusion.Nat Rev Neurosci. 2019 Jul;20(7):377. doi: 10.1038/s41583-019-0183-8. Nat Rev Neurosci. 2019. PMID: 31092911 No abstract available.
-
Restoring Activity of Pig Brain Cells After Death Does not Invalidate the Determination of Death by Neurologic Criteria or Undermine the Propriety of Organ Donation After Death.Transplantation. 2019 Jul;103(7):1295-1297. doi: 10.1097/TP.0000000000002782. Transplantation. 2019. PMID: 31107825 No abstract available.
-
[Restoration of cellular function in the brain after death].Medicina (B Aires). 2019;79(6):520-521. Medicina (B Aires). 2019. PMID: 31829958 Spanish. No abstract available.
-
Nature's 10: Ten people who mattered in science in 2019.Nature. 2019 Dec;576(7787):361-372. doi: 10.1038/d41586-019-03749-0. Nature. 2019. PMID: 31848484 No abstract available.
-
Death and Irreversibility.Camb Q Healthc Ethics. 2020 Jul;29(3):334-336. doi: 10.1017/S0963180120000043. Camb Q Healthc Ethics. 2020. PMID: 32484139 No abstract available.
-
Pig organs partially revived in dead animals - researchers are stunned.Nature. 2022 Aug;608(7922):247-248. doi: 10.1038/d41586-022-02112-0. Nature. 2022. PMID: 35922494 No abstract available.
References
-
- Kety SS Circulation and metabolism of the human brain. Brain Res Bull 50, 415–416 (1999). - PubMed
-
- Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res 24, 321–329 (1999). - PubMed
-
- Wagner S. R. t. & Lanier WL Metabolism of glucose, glycogen, and high-energy phosphates during complete cerebral ischemia. A comparison of normoglycemic, chronically hyperglycemic diabetic, and acutely hyperglycemic nondiabetic rats. Anesthesiology 81, 1516–1526 (1994). - PubMed
-
- Hoxworth JM, Xu K., Zhou Y., Lust WD & LaManna JC Cerebral metabolic profile, selective neuron loss, and survival of acute and chronic hyperglycemic rats following cardiac arrest and resuscitation. Brain Res 821, 467–479 (1999). - PubMed
Supplemental Discussion References
-
- Gilboe DD, Cotanch WW & Glover MB Extracorporeal Perfusion of the Isolated Head of a Dog. Nature 202, 399–400 (1964). - PubMed
-
- White RJ, Albin MS & Verdura J. Preservation of Viability in the Isolated Monkey Brain Utilizing a Mechanical Extracorporeal Circulation. Nature 202, 1082–1083 (1964). - PubMed
-
- von Bohlen und Halbach O. The isolated mammalian brain: an in vivo preparation suitable for pathway tracing. Eur J Neurosci 11, 1096–1100 (1999). - PubMed
-
- Muhlethaler M., de Curtis M., Walton K. & Llinas R. The isolated and perfused brain of the guinea-pig in vitro. Eur J Neurosci 5, 915–926 (1993). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

