Discovery of 1,4-Naphthoquinones as a New Class of Antiproliferative Agents Targeting GPR55
- PMID: 30996770
- PMCID: PMC6466525
- DOI: 10.1021/acsmedchemlett.8b00333
Discovery of 1,4-Naphthoquinones as a New Class of Antiproliferative Agents Targeting GPR55
Abstract
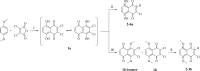
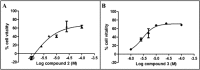
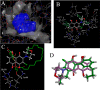
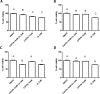
A new series of 1,4-naphthoquinones, bearing various cyclic and aliphatic amines on C2, was designed and synthesized to identify antiproliferative agents for triple-negative breast cancer, which represents a clinical challenge without targeted therapies. Among naphthoquinones, 2a and 3a inhibited the proliferation of MDA-MB-231 cells (EC50 = 1.6 and 2.7 μM, respectively), compared to primary human breast cells MCF10A. Furthermore, they did not affect the viability of peripheral blood mononuclear cells (PBMC), suggesting their potential safer use for cancer treatment. Recently, correlations have emerged between the expression of G protein-coupled receptor 55 (GPR55) and both triple-negative breast cancer development and invasion, making it a promising target for the development of targeted therapies. Based on this evidence, molecular docking studies supported the hypothesis of binding to GPR55, and pharmacological tests suggested that compound 3a could exert its antiproliferative activity acting as a GPR55 inverse agonist.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

