Discovery of Stereospecific PARP-1 Inhibitor Isoindolinone NMS-P515
- PMID: 30996792
- PMCID: PMC6466814
- DOI: 10.1021/acsmedchemlett.8b00569
Discovery of Stereospecific PARP-1 Inhibitor Isoindolinone NMS-P515
Abstract
Poly(ADP-ribose) polymerase-1 (PARP-1) is an enzyme involved in signaling and repair of DNA single strand breaks. PARP-1 employs NAD+ to modify substrate proteins via the attachment of poly(ADP-ribose) chains. PARP-1 is a well established target in oncology, as testified by the number of marketed drugs (e.g., Lynparza, Rubraca, Zejula, and Talzenna) used for the treatment of ovarian, breast, and prostate tumors. Efforts in investigating an uncharted region of the previously identified isoindolinone carboxamide series delivered (S)-13 (NMS-P515), a potent inhibitor of PARP-1 both in biochemical (K d: 0.016 μM) and cellular (IC50: 0.027 μM) assays. Cocrystal structure allowed explaining NMS-P515 stereospecific inhibition of the target. After having ruled out potential loss of enantiopurity in vitro and in vivo, NMS-P515 was synthesized in an asymmetric fashion. NMS-P515 ADME profile and its antitumor activity in a mouse xenograft cancer model render the compound eligible for further optimization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


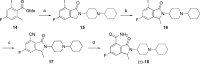
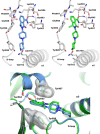

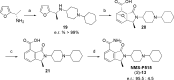
References
-
- Di Girolamo M.; Fabrizio G. The ADP-Ribosyl-Transferases Diphtheria Toxin-Like (ARTDs) Family: An Overview. Challenges 2018, 9, 24.10.3390/challe9010024. - DOI
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

