From nano-balls to nano-bowls
- PMID: 30996872
- PMCID: PMC6429618
- DOI: 10.1039/c8sc05471a
From nano-balls to nano-bowls
Abstract
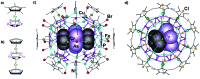
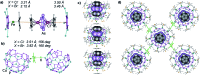
Pentaphosphaferrocene [Cp*Fe(η5-P5)] in combination with Cu(i) halides is capable of a template-directed synthesis of fullerene-like spheres. Herein, we present the use of a triple decker complex as template that leads to the formation of unprecedented 'nano-bowls'. These spherical domes resemble the truncated fullerenes I h-C80 and represent a novel spherical arrangement in the chemistry of spherical molecules.
Figures

References
-
- Bosch M., Yuan S., Rutledge W., Zhou H.-C. Acc. Chem. Res. 2017;50:857–865. - PubMed
- Manna B., Desai A. V., Ghosh S. K. Dalton Trans. 2016;45:4060–4072. - PubMed
- Kreno L. E., Leong K., Farha O. K., Allendorf M., Van Duyne R. P., Hupp J. T. Chem. Rev. 2012;112:1105. - PubMed
- Farha O. K., Hupp J. T. Acc. Chem. Res. 2010;43:1166. - PubMed
- Janiak C., Vieth J. K. New J. Chem. 2010;34:2366.
- Rowsell J. L. C., Yaghi O. M. Microporous Mesoporous Mater. 2004;73:3.
-
- Jiao J., Tan C., Li Z., Liu Y., Han X., Cui Y. J. Am. Chem. Soc. 2018;140:2251–2259. - PubMed
- Bestgen S., Fuhr O., Breitung B., Chakravadhanula V. S. K., Guthausen G., Hennrich F., Yu W., Kappes M. M., Roesky P. W., Fenske D. Chem. Sci. 2017;8:2235–2240. - PMC - PubMed
- Uchida J., Yoshio M., Sato S., Yokoyama H., Fujita M., Kato T. Angew. Chem., Int. Ed. 2017;56:14085–14089. - PubMed
- Roberts D. A., Pilgrim B. S., Nitschke J. R. Chem. Soc. Rev. 2018;47:626–644. - PubMed
- Sakthivel N. A., Theivendran S., Ganeshraj V., Oliver A. G., Dass A. J. Am. Chem. Soc. 2017;139:15450–15459. - PubMed
- Mitra T., Jelfs K. E., Schmidtmann M., Ahmed A., Chong S. Y., Adams D. J., Cooper A. I. Nat. Chem. 2013;5:276. - PubMed
- Saalfrank R. W., Scheurer A. Top. Curr. Chem. 2012;319:125. - PubMed
- Rizzuto F. J., Nitschke J. R. Nat. Chem. 2017;9:903–908. - PubMed
- Tiefenbacher K., Ajami D., Rebek J. Angew. Chem., Int. Ed. 2011;50:11805. - PMC - PubMed
- Qin L., Zhou G.-J., Yu Y.-Z., Nojiri H., Schröder C., Winpenny R. E. P., Zheng Y.-Z. J. Am. Chem. Soc. 2017;139:16405–16411. - PubMed
-
- Scherer O. J., Brück T. Angew. Chem., Int. Ed. Engl. 1987;26:59–61.
- Dielmann F., Merkle R., Heinl S., Scheer M. Z. Naturforsch., B: J. Chem. Sci. 2009;64:3–10.
- Heinl S., Balázs G., Scheer M. Phosphorus, Sulfur Silicon Relat. Elem. 2014;189:924–932.
-
- Heinl S., Peresypkina E. V., Virovets A. V., Scheer M. Angew. Chem., Int. Ed. 2015;54:13431. - PMC - PubMed
- Dielmann F., Fleischmann M., Heindl C., Peresypkina E. V., Virovets A. V., Gschwind R. M., Scheer M. Chem.–Eur. J. 2015;21:6208. - PMC - PubMed
- Dielmann F., Heindl C., Hastreiter F., Peresypkina E. V., Virovets A. V., Gschwind R. M., Scheer M. Angew. Chem., Int. Ed. 2014;53:13605. - PMC - PubMed
- Schindler A., Heindl C., Balázs G., Groeger C., Virovets A. V., Peresypkina E. V., Scheer M. Chem.–Eur. J. 2012;18:829. - PubMed
- Scheer M., Schindler A., Groeger C., Virovets A. V., Peresypkina E. V. Angew. Chem., Int. Ed. 2009;48:5046. - PubMed
- Scheer M., Schindler A., Merkle R., Johnson B. P., Linseis M., Winter R., Anson C. E., Virovets A. V. J. Am. Chem. Soc. 2007;129:13386. - PubMed
- Bai J., Virovets A. V., Scheer M. Science. 2003;300:781. - PubMed
-
- Peresypkina E. V., Heindl C., Virovets A., Mädl E., Brake H., Scheer M. Chem.–Eur. J. 2018;24:2503. - PMC - PubMed
- Heindl C., Peresypkina E., Virovets A. V., Bushmarinov I. S., Medvedev M. G., Krämer B., Dittrich B., Scheer M. Angew. Chem., Int. Ed. 2017;56:13237. - PMC - PubMed
- Peresypkina E., Heindl C., Virovets A. and Scheer M., Inorganic Superspheres, in Clusters – Contemporary Insight in Structure and Bonding, ed. S. Dehnen, 2016, vol. 174, pp. 321–373.
- Dielmann F., Peresypkina E. V., Krämer B., Hastreiter F., Johnson B. P., Zabel M., Heindl C., Scheer M. Angew. Chem., Int. Ed. 2016;55:14833. - PMC - PubMed
- Heindl C., Peresypkina E. V., Virovets A. V., Kremer W., Scheer M. J. Am. Chem. Soc. 2015;137:10938. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

