Transition-metal-free α-arylation of oxindoles vi a visible-light-promoted electron transfer
- PMID: 30996886
- PMCID: PMC6427940
- DOI: 10.1039/c8sc05170d
Transition-metal-free α-arylation of oxindoles vi a visible-light-promoted electron transfer
Abstract
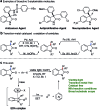
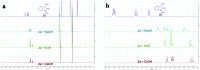
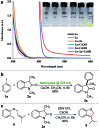
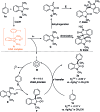
An operationally simple photochemical strategy for the direct arylation of oxindoles with (hetero)aryl halides in the absence of both transition metals and photoredox catalysts has been developed. The reaction provides an efficient way to construct various 3-aryloxindole building blocks of pharmaceutical interest at ambient temperature by using household compact fluorescent light (CFL) bulbs as the light source. Preliminarily, mechanistic studies revealed that the intermolecular electron transfer relied on the formation of photon-absorbing electron-donor-acceptor (EDA) complexes between electron-rich oxindole enolates and electron-deficient (hetero)aryl halides, and a radical chain mechanism was operative.
Figures
Similar articles
-
Dimsyl Anion Enables Visible-Light-Promoted Charge Transfer in Cross-Coupling Reactions of Aryl Halides.Adv Synth Catal. 2022 Jan 18;364(2):420-425. doi: 10.1002/adsc.202101052. Epub 2021 Oct 15. Adv Synth Catal. 2022. PMID: 37197314 Free PMC article.
-
Visible Light Mediated Photoredox Catalytic Arylation Reactions.Acc Chem Res. 2016 Aug 16;49(8):1566-77. doi: 10.1021/acs.accounts.6b00229. Epub 2016 Aug 2. Acc Chem Res. 2016. PMID: 27482835
-
Visible-Light-Promoted C-S Cross-Coupling via Intermolecular Charge Transfer.J Am Chem Soc. 2017 Oct 4;139(39):13616-13619. doi: 10.1021/jacs.7b07390. Epub 2017 Sep 19. J Am Chem Soc. 2017. PMID: 28910097 Free PMC article.
-
From α-arylation of olefins to acylation with aldehydes: a journey in regiocontrol of the Heck reaction.Acc Chem Res. 2011 Aug 16;44(8):614-26. doi: 10.1021/ar200053d. Epub 2011 May 25. Acc Chem Res. 2011. PMID: 21612205 Review.
-
Visible-Light Photocatalytic Reduction of Aryl Halides as a Source of Aryl Radicals.Molecules. 2022 Aug 23;27(17):5364. doi: 10.3390/molecules27175364. Molecules. 2022. PMID: 36080129 Free PMC article. Review.
Cited by
-
Synthetic reactions driven by electron-donor-acceptor (EDA) complexes.Beilstein J Org Chem. 2021 Apr 6;17:771-799. doi: 10.3762/bjoc.17.67. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33889219 Free PMC article. Review.
-
Photo-induced imino functionalizations of alkenes via intermolecular charge transfer.Chem Sci. 2023 Sep 25;14(40):11170-11179. doi: 10.1039/d3sc03667g. eCollection 2023 Oct 18. Chem Sci. 2023. PMID: 37860665 Free PMC article.
-
α-C(sp3)-H Arylation of Cyclic Carbonyl Compounds.Nat Prod Bioprospect. 2021 Aug;11(4):379-404. doi: 10.1007/s13659-021-00312-1. Epub 2021 Jun 7. Nat Prod Bioprospect. 2021. PMID: 34097248 Free PMC article. Review.
-
Visible-light-induced intramolecular charge transfer in the radical spirocyclisation of indole-tethered ynones.Chem Sci. 2019 Dec 13;11(5):1353-1360. doi: 10.1039/c9sc05311e. Chem Sci. 2019. PMID: 34123259 Free PMC article.
-
Electrochemical Umpolung C-H Functionalization of Oxindoles.J Org Chem. 2022 Jan 7;87(1):606-612. doi: 10.1021/acs.joc.1c02616. Epub 2021 Dec 28. J Org Chem. 2022. PMID: 34962127 Free PMC article.
References
-
- Feng Z., Yun-Lin L., Jian Z. Adv. Synth. Catal. 2010;352:1381.
- Shen K., Liu X., Lin L., Feng X. Chem. Sci. 2012;3:327.
- Russel J. S., in Heterocyclic Scaffolds II: Reactions and Applications of Indoles, ed. G. W. Gribble, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, p. 397, 10.1007/7081_2010_50. - DOI
-
- Natarajan A., Guo Y., Harbinski F., Fan Y.-H., Chen H., Luus L., Diercks J., Aktas H., Chorev M., Halperin J. A. J. Med. Chem. 2004;47:4979. - PubMed
-
- Luk K.-C., So S.-S., Zhang J. and Zhang Z., WO 2006136606A2, 2006.
-
- Hewawasam P., Gribkoff V. K., Pendri Y., Dworetzky S. I., Meanwell N. A., Martinez E., Boissard C. G., Post-Munson D. J., Trojnacki J. T., Yeleswaram K., Pajor L. M., Knipe J., Gao Q., Perrone R., Starrett J. E. Bioorg. Med. Chem. Lett. 2002;12:1023. - PubMed
-
- Altman R. A., Hyde A. M., Huang X., Buchwald S. L. J. Am. Chem. Soc. 2008;130:9613. - PMC - PubMed
- Taylor A. M., Altman R. A., Buchwald S. L. J. Am. Chem. Soc. 2009;131:9900. - PMC - PubMed
- Li P., Buchwald S. L. Angew. Chem., Int. Ed. 2011;50:6396. - PubMed
- Xiao Z.-K., Yin H.-Y., Shao L.-X. Org. Lett. 2013;15:1254. - PubMed
- Durbin M. J., Willis M. C. Org. Lett. 2008;10:1413. - PubMed
- Reddy Panyam P. K., Ugale B., Gandhi T. J. Org. Chem. 2018;83:7622. - PubMed
- Mai C.-K., Sammons M. F., Sammakia T. Org. Lett. 2010;12:2306. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

