Electrochemical fluoromethylation triggered lactonizations of alkenes under semi-aqueous conditions
- PMID: 30996899
- PMCID: PMC6429606
- DOI: 10.1039/c9sc00100j
Electrochemical fluoromethylation triggered lactonizations of alkenes under semi-aqueous conditions
Abstract
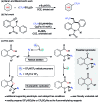
An electrochemical difluoromethylation triggered lactonization of alkenes was developed for the first time. This protocol employs readily prepared CF2HSO2Na as the difluoromethylating reagent, affording unprecedented CF2H-containing lactones in moderate yields. Moreover, with CF3SO2Na as the trifluoromethylating reagent, a wide array of CF3-containing lactones were obtained under additional supporting electrolyte- and catalyst-free conditions.
Figures
Similar articles
-
K2S2O8-Mediated Selective Trifluoromethylacylation and Trifluoromethylarylation of Alkenes under Transition-Metal-Free Conditions: Synthetic Scope and Mechanistic Studies.Org Lett. 2018 Oct 19;20(20):6520-6525. doi: 10.1021/acs.orglett.8b02846. Epub 2018 Oct 5. Org Lett. 2018. PMID: 30289263
-
Electrochemical Tri- and Difluoromethylation-Triggered Cyclization Accompanied by the Oxidative Cleavage of Indole Derivatives.Chemistry. 2021 Apr 12;27(21):6522-6528. doi: 10.1002/chem.202005368. Epub 2021 Mar 10. Chemistry. 2021. PMID: 33751675
-
Tetrafluoroisopropylation of alkenes and alkynes enabled by photocatalytic consecutive difluoromethylation with CF2HSO2Na.Nat Commun. 2024 Jul 6;15(1):5685. doi: 10.1038/s41467-024-50081-x. Nat Commun. 2024. PMID: 38971849 Free PMC article.
-
Visible Light Induced Oxydifluoromethylation of Styrenes with Difluoromethyltriphenylphosphonium Bromide.J Org Chem. 2016 Aug 19;81(16):7001-7. doi: 10.1021/acs.joc.6b00234. Epub 2016 May 23. J Org Chem. 2016. PMID: 27187524
-
Recent progress in photochemical radical di- and mono-fluoromethylation.Org Biomol Chem. 2019 Jun 5;17(22):5413-5419. doi: 10.1039/c9ob00734b. Org Biomol Chem. 2019. PMID: 31086872 Review.
Cited by
-
Inverse conjugate additions of acrylic amides and esters with F/Cl/O/N-nucleophiles and CF3+ reagents.Sci Adv. 2025 Feb 14;11(7):eadt2715. doi: 10.1126/sciadv.adt2715. Epub 2025 Feb 12. Sci Adv. 2025. PMID: 39937903 Free PMC article.
-
Electrochemical oxidative CF3 radical-induced lactonization and etherification of terminal and internal alkenes.RSC Adv. 2025 May 9;15(19):15302-15309. doi: 10.1039/d5ra01852h. eCollection 2025 May 6. RSC Adv. 2025. PMID: 40352397 Free PMC article.
-
Metal-Free Synthesis of N-Heterocycles via Intramolecular Electrochemical C-H Aminations.Front Chem. 2022 Jun 20;10:950635. doi: 10.3389/fchem.2022.950635. eCollection 2022. Front Chem. 2022. PMID: 35795218 Free PMC article. Review.
-
Synthesis of Difluoroarymethyl-Substituted Benzimidazo[2,1-a]isoquinolin-6(5H)-ones under Mild Conditions.ACS Omega. 2023 Feb 14;8(8):7517-7528. doi: 10.1021/acsomega.2c06689. eCollection 2023 Feb 28. ACS Omega. 2023. PMID: 36872989 Free PMC article.
-
Saturated oxygen and nitrogen heterocycles via oxidative coupling of alkyltrifluoroborates with alkenols, alkenoic acids and protected alkenylamines.Chem Sci. 2019 Aug 19;10(40):9265-9269. doi: 10.1039/c9sc02835h. eCollection 2019 Oct 28. Chem Sci. 2019. PMID: 32055311 Free PMC article.
References
-
- Kirsch P., Modern Fluoroorganic Chemistry: Synthesis Reactivity Applications, Wiley-VCH, Weinheim, 2004.
- Yamazaki T., Taguchi T. and Ojima I., Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, Great Britain, 2009.
-
-
For recent reviews on trifluoromethylations, see:
- Merino E., Nevado C. Chem. Soc. Rev. 2014;43:6598. - PMC - PubMed
- Charpentier J., Früh N., Togni A. Chem. Rev. 2015;115:650. - PubMed
- Pan X., Xia H., Wu J. Org. Chem. Front. 2016;3:1163.
- Song H.-X., Han Q.-Y., Zhao C.-L., Zhang C.-P. Green Chem. 2018;20:1662.
- Barata-Vallejo S., Cooke M.-V., Postigo A. ACS Catal. 2018;8:7287.
-
-
-
For recent examples on trifluoromethylations, see:
- Yang W., Ma D., Zhou Y., Dong X., Lin Z., Sun J.-W. Angew. Chem., Int. Ed. 2018;57:12097. - PubMed
- Valverde E., Kawamura S., Sekine D., Sodeoka M. Chem. Sci. 2018;9:7115. - PMC - PubMed
- Wang H., Xu Q., Yu S.-Y. Org. Chem. Front. 2018;5:2224.
- Mai B.-K., Szabó K.-J., Himo F. ACS Catal. 2018;8:4483.
- Borah A.-J., Shi Z.-Z. Chem. Commun. 2017;53:3945. - PubMed
- Han H.-S., Oh E.-Y., Jung Y.-S., Han S.-B. Org. Lett. 2018;20:1698. - PubMed
- Yang B., Yu D., Xu X.-H., Qing F.-L. ACS Catal. 2018;8:2839.
- Imiołek M., Karunanithy G., Ng W.-L., Baldwin A.-J., Gouverneur V., Davis B.-G. J. Am. Chem. Soc. 2018;140:1568. - PMC - PubMed
-
-
-
For recent reviews, see: . For selected recent works, see:
- Hu J.-B., Zhang W., Wang F. Chem. Commun. 2009:7465. - PubMed
- Belhomme M.-C., Besset T., Poisson T., Pannecoucke X. Chem.–Eur. J. 2015;21:12836. - PubMed
- Yerien D. E., Barata-Vallejo S., Postigo A. Chem.–Eur. J. 2017;23:14676. - PubMed
- Dilman A. D., Levin V. V. Acc. Chem. Res. 2018;51:1272. - PubMed
- Fier P. S., Hartwig J. F. J. Am. Chem. Soc. 2012;134:5524. - PMC - PubMed
- Fujiwara Y., Dixon J. A., Rodriguez R. A., Baxter R. D., Dixon D. D., Collins M. R., Blackmond D. G., Baran P. S. J. Am. Chem. Soc. 2012;134:1494. - PMC - PubMed
- Prakash G. K. S., Ganesh S. K., Jones J.-P., Kulkarni A., Masood K., Swabeck J. K., Olah G. A. Angew. Chem., Int. Ed. 2012;51:12090. - PubMed
- Iida T., Hashimoto R., Aikawa K., Ito S., Mikami K. Angew. Chem., Int. Ed. 2012;51:9535. - PubMed
- Mykhailiuk P. K. Angew. Chem., Int. Ed. 2015;54:6558. - PubMed
- Heine N.-B., Studer A. Org. Lett. 2017;19:4150. - PubMed
- Tung T. T., Christensen S. B., Nielsen J. Chem.–Eur. J. 2017;23:18125. - PubMed
-
LinkOut - more resources
Full Text Sources

