The stability enhancement factor beyond eight-electron shell closure in thiacalix[4]arene-protected silver clusters
- PMID: 30996924
- PMCID: PMC6430012
- DOI: 10.1039/c8sc03756f
The stability enhancement factor beyond eight-electron shell closure in thiacalix[4]arene-protected silver clusters
Abstract
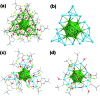
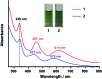
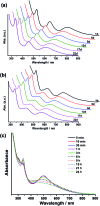
We report the synthesis and structures of two 34-atom metal nanoclusters, namely [Ag34(BTCA)3(C[triple bond, length as m-dash]CBu t )9(tfa)4(CH3OH)3]SbF6 and [AuAg33(BTCA)3(C[triple bond, length as m-dash]CBu t )9(tfa)4(CH3OH)3]SbF6, where H4BTCA is p-tert-butylthiacalix[4]arene and tfa is trifluoroacetate. Their compositions and structures have been determined by single-crystal X-ray structural analysis and ESI-MS. The cationic cluster consists of a centered icosahedron M@Ag12 (M = Ag or Au) core that is surrounded by 21 peripheral silver atoms. Surrounding protection is provided by four kinds of ligands, including three BTCA, nine t BuC[triple bond, length as m-dash]C, four tfa, and three methanol solvent ligands. It was found that the Ag5@BTCA μ5-coordination motif of thiacalixarene is critical for high stability of the title clusters, and extra stability enhancement can be achieved by doping a gold atom at the center of the silver cluster. This work suggests that coordination saturation should be taken into account in addition to electronic and geometric factors for analyzing metal nanocluster stabilities.
This journal is © The Royal Society of Chemistry 2019.
Figures



Similar articles
-
Thiacalix[4]arene: New protection for metal nanoclusters.Sci Adv. 2016 Aug 12;2(8):e1600323. doi: 10.1126/sciadv.1600323. eCollection 2016 Aug. Sci Adv. 2016. PMID: 27536724 Free PMC article.
-
All-Calixarene-Protected Silver Nanocluster with All Silver Atoms in a Face-Centered Cubic Arrangement.J Am Chem Soc. 2024 Sep 11;146(36):25101-25107. doi: 10.1021/jacs.4c08094. Epub 2024 Aug 28. J Am Chem Soc. 2024. PMID: 39196903
-
Ligand Engineering toward the Trade-Off between Stability and Activity in Cluster Catalysis.Angew Chem Int Ed Engl. 2022 Mar 7;61(11):e202116965. doi: 10.1002/anie.202116965. Epub 2022 Jan 24. Angew Chem Int Ed Engl. 2022. PMID: 35014157
-
Advancements in Atomically Precise Nanocluster Protected by Thiacalix[4]arene.Adv Mater. 2024 Nov;36(45):e2410054. doi: 10.1002/adma.202410054. Epub 2024 Sep 3. Adv Mater. 2024. PMID: 39226533 Review.
-
Insight into the Geometric and Electronic Structures of Gold/Silver Superatomic Clusters Based on Icosahedron M13 Units and Their Alloys.Chem Asian J. 2019 Oct 1;14(19):3222-3231. doi: 10.1002/asia.201900760. Epub 2019 Sep 3. Chem Asian J. 2019. PMID: 31368672 Review.
Cited by
-
Isomerization-induced enhancement of luminescence in Au28(SR)20 nanoclusters.Chem Sci. 2020 Jul 17;11(31):8176-8183. doi: 10.1039/d0sc01270j. Chem Sci. 2020. PMID: 34123088 Free PMC article.
-
Regulating the assembly and expansion of the silver cluster from the Ag37 to Ag46 nanowheel driven by heteroanions.Chem Sci. 2022 Dec 26;14(5):1138-1144. doi: 10.1039/d2sc06436g. eCollection 2023 Feb 1. Chem Sci. 2022. PMID: 36756341 Free PMC article.
-
A hierarchically assembled 88-nuclei silver-thiacalix[4]arene nanocluster.Nat Commun. 2020 Jan 16;11(1):308. doi: 10.1038/s41467-019-13682-5. Nat Commun. 2020. PMID: 31949133 Free PMC article.
-
Atomic-level engineering of single Ag1+ site distribution on titanium-oxo cluster surfaces to boost CO2 electroreduction.Chem Sci. 2025 Jan 22;16(16):6845-6852. doi: 10.1039/d4sc07186g. eCollection 2025 Apr 16. Chem Sci. 2025. PMID: 40110521 Free PMC article.
-
Synergistic effects of atomically precise Au-based bimetallic nanocluster on energy-related small molecule catalysis.Chem Sci. 2025 Apr 30;16(24):10642-10664. doi: 10.1039/d5sc01108f. eCollection 2025 Jun 18. Chem Sci. 2025. PMID: 40453807 Free PMC article. Review.
References
-
- Daniel M. C., Astruc D. Chem. Rev. 2004;104:293–346. - PubMed
-
- Jin R., Zeng C., Zhou M., Chen Y. Chem. Rev. 2016;116:10346–10413. - PubMed
-
- Parker J. F., Fields-Zinna C. A., Murray R. W. Acc. Chem. Res. 2010;43:1289–1296. - PubMed
-
- Schmid G. Chem. Soc. Rev. 2008;37:1909–1930. - PubMed
-
- Jin R. X., Zhao S., Xing Y., Jin R. CrystEngComm. 2016;18:3996–4005.
LinkOut - more resources
Full Text Sources
Research Materials

