A new route for the efficient metalation of unfunctionalized aromatics
- PMID: 30996927
- PMCID: PMC6429619
- DOI: 10.1039/c8sc04325f
A new route for the efficient metalation of unfunctionalized aromatics
Abstract
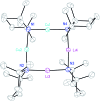
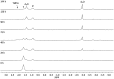
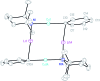
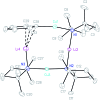
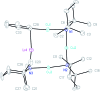
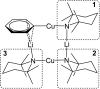
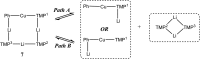
The synthesis and isolation of a novel bimetallic species formed by reacting two equivalents of TMPLi with CuCl in the presence of Et2O are reported. X-ray crystallography reveals the Et2O-free tetranuclear aggregate (TMPCu)2(TMPLi)2 1, which formally results from the catenation of dimers of TMPLi and TMPCu. NMR spectroscopy confirms that, upon dissolution in hydrocarbon media, the crystals fail to form a conventional Gilman cuprate dimer. Instead they exhibit a spectrum which is consistent with that recently proposed for an isomer of dimeric Gilman cuprate. Moreover, while pre-isolated Gilman cuprate is inert to benzene solvent, this new isomer smoothly affects aromatic deprotonation to give mainly Ph(TMP)3Cu2Li2 3, which is formally a heterodimer of Gilman cuprate TMPCu(μ-TMP)Li 2 and PhCu(μ-TMP)Li 4. Attempts to synthesise 3 through explicit combination of pre-isolated 2 and 4 were successful; additionally, this permitted the preparation of Ph(TMP)3Cu3Li 5 and Ph(TMP)3CuLi3 7 when 4 was combined in 1 : 2 ratios with TMPCu or TMPLi, respectively. 5 was characterised as metallacyclic in the solid-state, its structural features resembling those in 3 but with reduced Li-π interactions. It also proved possible to perform Cu/Li exchange on 5 (using t BuOCu) to give a novel mixed organo(amido)copper species Ph(TMP)3Cu4 6. Remarkably, the unprecedented reactivity of 1 towards benzene is reproduced by heating a 1 : 1 mixture of TMPLi and TMPCu in the same solvent; this gives predominantly 3. On the other hand, mixtures which are rich in either Cu or Li can lead to the selective in situ formation of 5 or 7. Though crystallographic data on 7 could not be obtained, DFT calculations accurately corroborated the observed structures of 3 and 5 and could be used to support 7 having the same structure type, albeit with enhanced Li-π interactions. This was consistent with NMR spectroscopic data. However, in contrast to 3 and 5, for which 2D NMR spectroscopy indicated only conformational changes, 7 was additionally found to exhibit fluxionality in a manner consistent with a dissociative process.
Figures




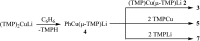
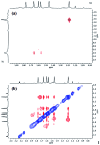
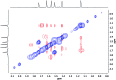
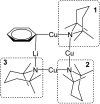

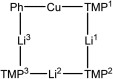
Similar articles
-
Metal exchange in lithiocuprates: implications for our understanding of structure and reactivity.Chem Sci. 2017 Jul 1;8(7):4904-4916. doi: 10.1039/c7sc01423f. Epub 2017 May 4. Chem Sci. 2017. PMID: 28959414 Free PMC article.
-
Extending motifs in lithiocuprate chemistry: unexpected structural diversity in thiocyanate complexes.Dalton Trans. 2016 Apr 14;45(14):6094-104. doi: 10.1039/c5dt03882k. Epub 2015 Nov 10. Dalton Trans. 2016. PMID: 26554572
-
Structural effects in lithiocuprate chemistry: the elucidation of reactive pentametallic complexes.Chemistry. 2014 Apr 1;20(14):3908-12. doi: 10.1002/chem.201304824. Epub 2014 Feb 19. Chemistry. 2014. PMID: 24550148 Free PMC article.
-
Avant-garde metalating agents: structural basis of alkali-metal-mediated metalation.Acc Chem Res. 2009 Jun 16;42(6):743-55. doi: 10.1021/ar800254y. Acc Chem Res. 2009. PMID: 19348412
-
New avenues in the directed deprotometallation of aromatics: recent advances in directed cupration.Dalton Trans. 2014 Oct 14;43(38):14181-14203. doi: 10.1039/c4dt01130a. Dalton Trans. 2014. PMID: 24919957 Review.
Cited by
-
Lipshutz-type bis(amido)argentates for directed ortho argentation.Chem Sci. 2020 Jan 7;11(7):1855-1861. doi: 10.1039/c9sc06060j. Chem Sci. 2020. PMID: 34123279 Free PMC article.
References
-
- Kondo Y., Shilai M., Uchiyama M., Sakamoto T. J. Am. Chem. Soc. 1999;121:3539–3540.
-
- Mulvey R. E. Dalton Trans. 2013;42:6676–6693. - PubMed
-
- Mulvey R. E., Blair V. L., Clegg W., Kennedy A. R., Klett J., Russo L. Nat. Chem. 2010;2:588–591. - PubMed
- Martínez-Martínez A. J., Kennedy A. R., Mulvey R. E., O'Hara C. T. Science. 2014;346:834–837. - PubMed
- Brikci-Nigassa N. M., Bhentabed-Ababsa G., Erb W., Mongin F. Synthesis. 2018:3615–3633.
- Uzelac M., Mulvey R. E. Chem.–Eur. J. 2018;24:7786–7793. - PubMed
-
- Harrison-Marchand A., Mongin F. Chem. Rev. 2013;113:7470–7562. - PubMed
- Mongin F., Harrison-Marchand A. Chem. Rev. 2013;113:7563–7727. - PubMed
- Davin L., McLellan R., Kennedy A. R., Hevia E. Chem. Commun. 2017;53:11650–11653. - PubMed
- Martínez-Martínez A. J., Justice S., Fleming B. J., Kennedy A. R., Oswald I. D. H., O'Hara C. T. Sci. Adv. 2017;3:e1700832. - PMC - PubMed
- Stevens M. A., Hashim F. H., Gwee E. S. H., Izgorodina E. I., Mulvey R. E. and Blair V. L., Chem.–Eur. J., 2018, 24, 15669–15677.. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

