Facile N-functionalization and strong magnetic communication in a diuranium(v) bis-nitride complex
- PMID: 30996946
- PMCID: PMC6438153
- DOI: 10.1039/c8sc05721d
Facile N-functionalization and strong magnetic communication in a diuranium(v) bis-nitride complex
Erratum in
-
Correction: Facile N-functionalization and strong magnetic communication in a diuranium(v) bis-nitride complex.Chem Sci. 2019 Mar 6;10(12):3687. doi: 10.1039/c9sc90049g. eCollection 2019 Mar 28. Chem Sci. 2019. PMID: 30997905 Free PMC article.
Abstract
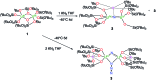
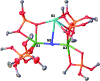
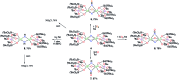
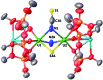
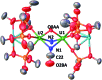
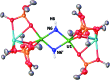
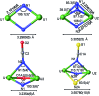
Uranium nitride complexes are of high interest because of their ability to effect dinitrogen reduction and functionalization and to promote magnetic communication, but studies of their properties and reactivity remain rare. Here we have prepared in 73% yield the diuranium(v) bis-nitride complex [K2{[U(OSi(O t Bu)3)3]2(μ-N)2}], 4, from the thermal decomposition of the nitride-, azide-bridged diuranium(iv) complex [K2{[U(OSi(O t Bu)3)3]2(μ-N)(μ-N3)}], 3. The bis-nitride 4 reacts in ambient conditions with 1 equiv. of CS2 and 1 equiv. of CO2 resulting in N-C bond formation to afford the diuranium(v) complexes [K2{[U(OSi(O t Bu)3)3]2(μ-N)(μ-S)(μ-NCS)}], 5 and [K2{[U(OSi(O t Bu)3)3]2(μ-N)(μ-O)(μ-NCO)}], 6, respectively. Both nitrides in 4 react with CO resulting in oxidative addition of CO to one nitride and CO cleavage by the second nitride to afford the diuranium(iv) complex [K2{[U(OSi(O t Bu)3)3]2(μ-CN)(μ-O)(μ-NCO)}], 7. Complex 4 also effects the remarkable oxidative cleavage of H2 in mild conditions to afford the bis-imido bridged diuranium(iv) complex [K2{[U(OSi(O t Bu)3)3]2(μ-NH)2}], 8 that can be further protonated to afford ammonia in 73% yield. Complex 8 provides a good model for hydrogen cleavage by metal nitrides in the Haber-Bosch process. The measured magnetic data show an unusually strong antiferromagnetic coupling between uranium(v) ions in the complexes 4 and 6 with Neel temperatures of 77 K and 60 K respectively, demonstrating that nitrides are attractives linkers for promoting magnetic communication in uranium complexes.
Figures




References
-
- Nishibayashi Y.,in Nitrogen Fixation, ed. Y. Nishibayashi, 2017, vol. 60, pp. V–V.
- Schlogl R. Angew. Chem., Int. Ed. 2003;42:2004–2008. - PubMed
- Klopsch I., Yuzik-Klimova E. Y. and Schneider S., in Nitrogen Fixation, ed. Y. Nishibayashi, 2017, vol. 60, pp. 71–112.
- Fryzuk M. D. Science. 2013;340:1530–1531. - PubMed
- Hoffman B. M., Lukoyanov D., Dean D. R., Seefeldt L. C. Acc. Chem. Res. 2013;46:587–595. - PMC - PubMed
- Jia H. P., Quadrelli E. A. Chem. Soc. Rev. 2014;43:547–564. - PubMed
-
- Schoffel J., Rogachev A. Y., George S. D., Burger P. Angew. Chem., Int. Ed. 2009;48:4734–4738. - PubMed
- Shaver M. P., Fryzuk M. D. Adv. Synth. Catal. 2003;345:1061–1076.
- Brown S. D., Mehn M. P., Peters J. C. J. Am. Chem. Soc. 2005;127:13146–13147. - PubMed
- Askevold B., Nieto J. T., Tussupbayev S., Diefenbach M., Herdtweck E., Holthausen M. C., Schneider S. Nat. Chem. 2011;3:532–537. - PubMed
- Schendzielorz F. S., Finger M., Volkmann C., Wurtele C., Schneider S. Angew. Chem., Int. Ed. 2016;55:11417–11420. - PubMed
- Scepaniak J. J., Bontchev R. P., Johnson D. L., Smith J. M. Angew. Chem., Int. Ed. 2011;50:6630–6633. - PubMed
- Scepaniak J. J., Vogel C. S., Khusniyarov M. M., Heinemann F. W., Meyer K., Smith J. M. Science. 2011;331:1049–1052. - PubMed
- Betley T. A., Peters J. C. J. Am. Chem. Soc. 2004;126:6252–6254. - PubMed
- Brown S. D., Peters J. C. J. Am. Chem. Soc. 2005;127:1913–1923. - PubMed
- Buss J. A., Cheng C., Agapie T. Angew. Chem., Int. Ed. 2018;57:9670–9674. - PubMed
- MacLeod K. C., McWilliams S. F., Mercado B. Q., Holland P. L. Chem. Sci. 2016;7:5736–5746. - PMC - PubMed
-
- Silvia J. S., Cummins C. C. J. Am. Chem. Soc. 2009;131:446–447. - PubMed
- Falcone M., Kefalidis C. E., Scopelliti R., Maron L., Mazzanti M. Angew. Chem., Int. Ed. 2016;55:12290–12294. - PubMed
- Falcone M., Chatelain L., Scopelliti R., Zivkovic I., Mazzanti M. Nature. 2017;547:332–335. - PubMed
- Cleaves P. A., King D. M., Kefalidis C. E., Maron L., Tuna F., McInnes E. J. L., McMaster J., Lewis W., Blake A. J., Liddle S. T. Angew. Chem., Int. Ed. 2014;53:10412–10415. - PMC - PubMed
-
- Cleaves P. A., Kefalidis C. E., Gardner B. M., Tuna F., McInnes E. J. L., Lewis W., Maron L., Liddle S. T. Chem. –Eur. J. 2017;23:2950–2959. - PubMed
- Ishida Y., Kawaguchi H. J. Am. Chem. Soc. 2014;136:16990–16993. - PubMed
- Tran B. L., Pink M., Gao X. F., Park H., Mindiola D. J. J. Am. Chem. Soc. 2010;132:1458–1459. - PubMed
- Cozzolino A. F., Silvia J. S., Lopez N., Cummins C. C. J. Chem. Soc., Dalton Trans. 2014;43:4639–4652. - PubMed
- Brask J. K., Dura-Vila V., Diaconescu P. L., Cummins C. C. Chem. Commun. 2002:902–903. - PubMed
- Silvia J. S., Cummins C. C. J. Am. Chem. Soc. 2010;132:2169–2170. - PubMed
- Falcone M., Chatelain L., Mazzanti M. Angew. Chem., Int. Ed. 2016;55:4074–4078. - PubMed
- Semproni S. P., Chirik P. J. Angew. Chem., Int. Ed. 2013;52:12965–12969. - PubMed
- Semproni S. P., Chirik P. J. J. Am. Chem. Soc. 2013;135:11373–11383. - PubMed
- Smith J. M. Prog. Inorg. Chem. 2014;58:417–470.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

