Ultraspecific live imaging of the dynamics of zebrafish neutrophil granules by a histopermeable fluorogenic benzochalcone probe
- PMID: 30996961
- PMCID: PMC6432617
- DOI: 10.1039/c8sc05593a
Ultraspecific live imaging of the dynamics of zebrafish neutrophil granules by a histopermeable fluorogenic benzochalcone probe
Abstract
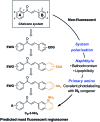
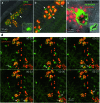
Neutrophil granules (NGs) are key components of the innate immune response and mark the development of neutrophilic granulocytes in mammals. However, there has been no specific fluorescent vital stain up to now to monitor their dynamics within a whole live organism. We rationally designed a benzochalcone fluorescent probe (HAB) featuring high tissue permeability and optimal photophysics such as elevated quantum yield, pronounced solvatochromism and target-induced fluorogenesis. Phenotypic screening identified HAB as the first cell- and organelle-specific small-molecule fluorescent tracer of NGs in live zebrafish larvae, with no labeling of other cell types or organelles. HAB staining was independent of the state of neutrophil activation, labeling NGs of both resting and phagocytically active neutrophils with equal specificity. By high-resolution live imaging, we documented the dynamics of HAB-stained NGs during phagocytosis. Upon zymosan injection, labeled NGs were rapidly recruited to the forming phagosomes. Despite being a reversible ligand, HAB could not be displaced by high concentrations of pharmacologically relevant competing chalcones, indicating that this specific labeling was the result of the HAB's precise physicochemical signature rather than a general feature of chalcones. However, one of the competitors was discovered as a promising interstitial fluorescent tracer illuminating zebrafish histology, similarly to BODIPY-ceramide. As a yellow-emitting histopermeable vital stain, HAB functionally and spectrally complements most genetically incorporated fluorescent tags commonly used in live zebrafish biology, holding promise for the study of neutrophil-dependent responses relevant to human physiopathology such as developmental defects, inflammation and infection. Furthermore, HAB intensely labeled isolated live human neutrophils at the level of granulated subcellular structures consistent with human NGs, suggesting that the labeling of NGs by HAB is not restricted to the zebrafish model but also relevant to mammalian systems.
Figures







Similar articles
-
Targeting chalcone binding sites in living Leishmania using a reversible fluorogenic benzochalcone probe.Biomed Pharmacother. 2022 May;149:112784. doi: 10.1016/j.biopha.2022.112784. Epub 2022 Mar 14. Biomed Pharmacother. 2022. PMID: 35299122
-
Confocal microscopic analysis of morphogenetic movements.Methods Cell Biol. 1999;59:179-204. doi: 10.1016/s0091-679x(08)61826-9. Methods Cell Biol. 1999. PMID: 9891361 Review.
-
Molecular Insights Into Neutrophil Biology From the Zebrafish Perspective: Lessons From CD18 Deficiency.Front Immunol. 2021 Sep 7;12:677994. doi: 10.3389/fimmu.2021.677994. eCollection 2021. Front Immunol. 2021. PMID: 34557186 Free PMC article.
-
Investigations of phagosomes, mitochondria, and acidic granules in human neutrophils using fluorescent probes.Cytometry B Clin Cytom. 2003 Jan;51(1):21-9. doi: 10.1002/cyto.b.10003. Cytometry B Clin Cytom. 2003. PMID: 12500294
-
Inflammatory Responses during Tumour Initiation: From Zebrafish Transgenic Models of Cancer to Evidence from Mouse and Man.Cells. 2020 Apr 20;9(4):1018. doi: 10.3390/cells9041018. Cells. 2020. PMID: 32325966 Free PMC article. Review.
Cited by
-
A modular aldol approach for internal fluorescent molecular rotor chalcone surrogates for DNA biosensing applications.Chem Sci. 2023 Apr 11;14(18):4832-4844. doi: 10.1039/d3sc00772c. eCollection 2023 May 10. Chem Sci. 2023. PMID: 37181758 Free PMC article.
-
A Photoalkylative Fluorogenic Probe of Guttiferone A for Live Cell Imaging and Proteome Labeling in Plasmodium falciparum.Molecules. 2020 Nov 4;25(21):5139. doi: 10.3390/molecules25215139. Molecules. 2020. PMID: 33158263 Free PMC article.
References
-
- Masud S., Torraca V., Meijer A. H. Curr. Top. Dev. Biol. 2017;124:277–329. - PubMed
-
- Lieschke G. J., Oates A. C., Crowhurst M. O., Ward A. C., Layton J. E. Blood. 2001;98:3087–3096. - PubMed
-
- Traver D., Herbomel P., Patton E. E., Murphey R. D., Yoder J. A., Litman G. W., Catic A., Amemiya C. T., Zon L. I., Trede N. S. Adv. Immunol. 2003;81:253–330. - PubMed
-
- Herbomel P., Thisse B., Thisse C. Development. 1999;126:3735–3745. - PubMed
-
- Le Guyader D., Redd M. J., Colucci-Guyon E., Murayama E., Kissa K., Briolat V., Mordelet E., Zapata A., Shinomiya H., Herbomel P. Blood. 2008;111:132–141. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

