Low overpotential water oxidation at neutral pH catalyzed by a copper(ii) porphyrin
- PMID: 30996977
- PMCID: PMC6419937
- DOI: 10.1039/c8sc04529a
Low overpotential water oxidation at neutral pH catalyzed by a copper(ii) porphyrin
Abstract
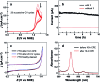
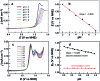
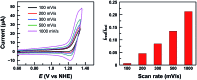
Low overpotential water oxidation under mild conditions is required for new energy conversion technologies with potential application prospects. Extensive studies on molecular catalysis have been performed to gain fundamental knowledge for the rational designing of cheap, efficient and robust catalysts. We herein report a water-soluble CuII complex of tetrakis(4-N-methylpyridyl)porphyrin (1), which catalyzes the oxygen evolution reaction (OER) in neutral aqueous solutions with small overpotentials: the onset potential of the catalytic water oxidation wave measured at current density j = 0.10 mA cm-2 is 1.13 V versus a normal hydrogen electrode (NHE), which corresponds to an onset overpotential of 310 mV. Constant potential electrolysis of 1 at neutral pH and at 1.30 V versus NHE displayed a substantial and stable current for O2 evolution with a faradaic efficiency of >93%. More importantly, in addition to the 4e water oxidation to O2 at neutral pH, 1 can catalyze the 2e water oxidation to H2O2 in acidic solutions. The produced H2O2 is detected by rotating ring-disk electrode measurements and by the sodium iodide method after bulk electrolysis at pH 3.0. This work presents an efficient and robust Cu-based catalyst for water oxidation in both neutral and acidic solutions. The observation of H2O2 during water oxidation catalysis is rare and will provide new insights into the water oxidation mechanism.
Figures



Similar articles
-
Water Oxidation at Neutral pH using a Highly Active Copper-Based Electrocatalyst.ChemSusChem. 2020 Sep 18;13(18):5088-5099. doi: 10.1002/cssc.202001444. Epub 2020 Aug 10. ChemSusChem. 2020. PMID: 32667741
-
Electrocatalytic Water Oxidation by a Water-Soluble Nickel Porphyrin Complex at Neutral pH with Low Overpotential.Inorg Chem. 2015 Jun 1;54(11):5604-13. doi: 10.1021/acs.inorgchem.5b00924. Epub 2015 May 18. Inorg Chem. 2015. PMID: 25985258
-
Electrocatalytic water oxidation using a chair-like tetranuclear copper(ii) complex in a neutral aqueous solution.Dalton Trans. 2016 Aug 9;45(32):12685-90. doi: 10.1039/c6dt01891b. Dalton Trans. 2016. PMID: 27445118
-
A Copper Porphyrin-Based Conjugated Mesoporous Polymer-Derived Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution.ChemSusChem. 2016 Sep 8;9(17):2365-73. doi: 10.1002/cssc.201600452. Epub 2016 Aug 17. ChemSusChem. 2016. PMID: 27530422
-
Optimizing the Synthetic Potential of O2: Implications of Overpotential in Homogeneous Aerobic Oxidation Catalysis.J Am Chem Soc. 2023 Aug 16;145(32):17515-17526. doi: 10.1021/jacs.3c02887. Epub 2023 Aug 3. J Am Chem Soc. 2023. PMID: 37534994 Free PMC article. Review.
Cited by
-
Homogeneous Catalysts Based on First-Row Transition-Metals for Electrochemical Water Oxidation.ChemSusChem. 2021 Jan 7;14(1):234-250. doi: 10.1002/cssc.202001876. Epub 2020 Oct 16. ChemSusChem. 2021. PMID: 32991076 Free PMC article. Review.
-
A homogeneous cobalt complex mediated electro and photocatalytic O2/H2O interconversion in neutral water.iScience. 2023 Oct 12;26(11):108189. doi: 10.1016/j.isci.2023.108189. eCollection 2023 Nov 17. iScience. 2023. PMID: 37920669 Free PMC article.
-
Understanding the factors governing the water oxidation reaction pathway of mononuclear and binuclear cobalt phthalocyanine catalysts.Chem Sci. 2022 Jul 8;13(30):8797-8803. doi: 10.1039/d2sc02213c. eCollection 2022 Aug 4. Chem Sci. 2022. PMID: 35975146 Free PMC article.
-
Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments.Chem Soc Rev. 2022 Jun 6;51(11):4583-4762. doi: 10.1039/d0cs01079k. Chem Soc Rev. 2022. PMID: 35575644 Free PMC article. Review.
-
Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction.Int J Mol Sci. 2022 May 27;23(11):6036. doi: 10.3390/ijms23116036. Int J Mol Sci. 2022. PMID: 35682721 Free PMC article. Review.
References
-
- Blakemore J. D., Crabtree R. H., Brudvig G. W. Chem. Rev. 2015;115:12974–13005. - PubMed
-
- Gust D., Moore T. A., Moore A. L. Acc. Chem. Res. 2009;42:1890–1898. - PubMed
-
- Zhang W., Lai W. Z., Cao R. Chem. Rev. 2017;117:3717–3797. - PubMed
-
- Joya K. S., Joya Y. F., Ocakoglu K., van de Krol R. Angew. Chem., Int. Ed. 2013;52:10426–10437. - PubMed
-
- Qi J., Zhang W., Cao R. Adv. Energy Mater. 2018;8:1701620.
LinkOut - more resources
Full Text Sources
Other Literature Sources

