Clopidogrel Pharmacogenetics
- PMID: 30998396
- PMCID: PMC6581205
- DOI: 10.1161/CIRCINTERVENTIONS.119.007811
Clopidogrel Pharmacogenetics
Abstract
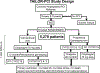
Common genetic variation in CYP2C19 (cytochrome P450, family 2, subfamily C, polypeptide 19) *2 and *3 alleles leads to a loss of functional protein, and carriers of these loss-of-function alleles when treated with clopidogrel have significantly reduced clopidogrel active metabolite levels and high on-treatment platelet reactivity resulting in increased risk of major adverse cardiovascular events, especially after percutaneous coronary intervention. The Food and Drug Administration has issued a black box warning advising practitioners to consider alternative treatment in CYP2C19 poor metabolizers who might receive clopidogrel and to identify such patients by genotyping. However, routine clinical use of genotyping for CYP2C19 loss-of-function alleles in patients undergoing percutaneous coronary intervention is not recommended by clinical guidelines because of lack of prospective evidence. To address this critical gap, TAILOR-PCI (Tailored Antiplatelet Initiation to Lessen Outcomes due to Decreased Clopidogrel Response After Percutaneous Coronary Intervention) is a large, pragmatic, randomized trial comparing point-of-care genotype-guided antiplatelet therapy with routine care to determine whether identifying CYP2C19 loss-of-function allele patients prospectively and prescribing alternative antiplatelet therapy is beneficial.
Keywords: clinical trial; clopidogrel; cytochrome P450 CYP2C19; drug labeling; genetics; humans; pharmacogenetics.
Figures
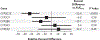


References
-
- Gandhi S, Zile B, Tan MK, Saranu J, Bucci C, Yan AT, Robertson P, Quantz MA, Letovsky E, Tanguay J-F, Dery J-P, Fitchett D, Madan M, Cantor WJ, Heffernan M, Natarajan MK, Wong GC, Welsh RC, Goodman SG. Increased uptake of guideline-recommended oral antiplatelet therapy: insights from the Canadian Acute Coronary Syndrome Reflective. Can J Cardiol. 2014;30:1725–1731. - PubMed
-
- Karve AM, Seth M, Sharma M, LaLonde T, Dixon S, Wohns D, Gurm HS. Contemporary use of ticagrelor in interventional practice (from Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Am J Cardiol. 2015;115:1502–1506. - PubMed
-
- Price MJ, Murray SS, Angiolillo DJ, Lillie E, Smith EN, Tisch RL, Schork NJ, Teirstein PS, Topol EJ. Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: The GIFT (Genotype Information and Functional Testing) Study. J Am Coll Cardiol. 2012;59:1928–1937. - PubMed
-
- Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome p450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. - PMC - PubMed
-
- Holmes DR Jr., Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56:321–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

