Aged marrow macrophages expand platelet-biased hematopoietic stem cells via Interleukin1B
- PMID: 30998506
- PMCID: PMC6542605
- DOI: 10.1172/jci.insight.124213
Aged marrow macrophages expand platelet-biased hematopoietic stem cells via Interleukin1B
Abstract
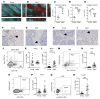
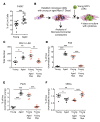
The bone marrow microenvironment (BMME) contributes to the regulation of hematopoietic stem cell (HSC) function, though its role in age-associated lineage skewing is poorly understood. Here we show that dysfunction of aged marrow macrophages (Mφs) directs HSC platelet-bias. Mφs from the marrow of aged mice and humans exhibited an activated phenotype, with increased expression of inflammatory signals. Aged marrow Mφs also displayed decreased phagocytic function. Senescent neutrophils, typically cleared by marrow Mφs, were markedly increased in aged mice, consistent with functional defects in Mφ phagocytosis and efferocytosis. In aged mice, Interleukin 1B (IL1B) was elevated in the bone marrow and caspase 1 activity, which can process pro-IL1B, was increased in marrow Mφs and neutrophils. Mechanistically, IL1B signaling was necessary and sufficient to induce a platelet bias in HSCs. In young mice, depletion of phagocytic cell populations or loss of the efferocytic receptor Axl expanded platelet-biased HSCs. Our data support a model wherein increased inflammatory signals and decreased phagocytic function of aged marrow Mφs induce the acquisition of platelet bias in aged HSCs. This work highlights the instructive role of Mφs and IL1B in the age-associated lineage-skewing of HSCs, and reveals the therapeutic potential of their manipulation as antigeronic targets.
Keywords: Aging; Bone marrow; Hematology; Hematopoietic stem cells.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

